Preparation method of palbociclib intermediate
An intermediate and solid technology, which is applied in the field of preparation of palbociclib intermediates, can solve the problems of unfavorable final product palbociclib product popularization, unfavorable sewage treatment, unfavorable recycling, etc., to achieve the benefits of three wastes treatment, Ease of handling and reduction of environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
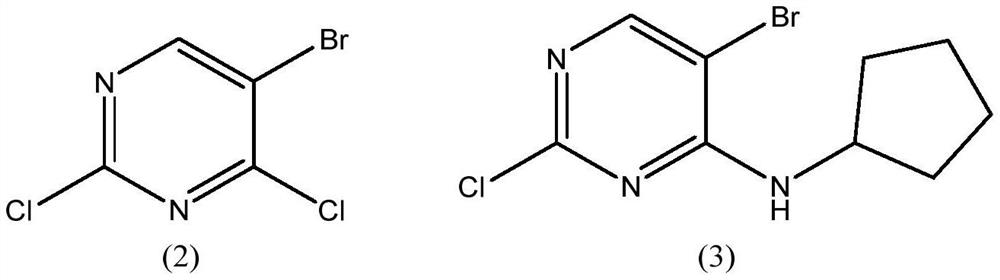
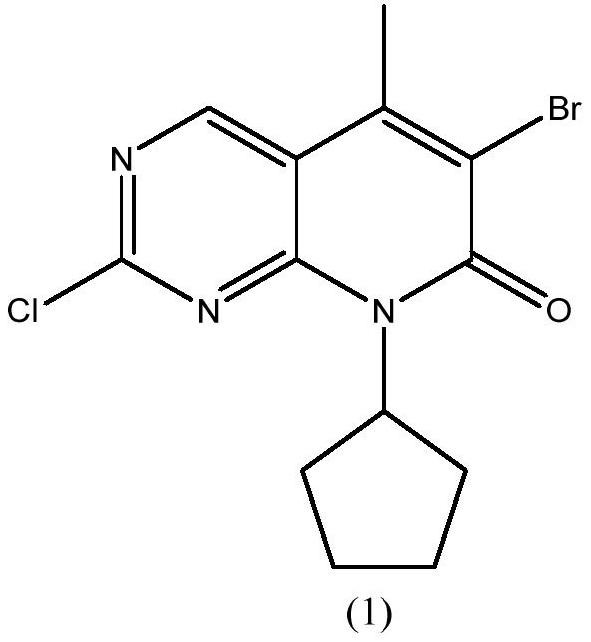
[0035] Example 1 Synthesis of 6-bromo-2-chloro-8-cyclopentyl-5-methylpyrido[2,3-D]pyrimidin-7(8H)-one
[0036] (1) Preparation of 5-bromo-2-chloro-N-cyclopentamine-4 amine
[0037] Add 228 grams (1.0mol) of 5-bromo-2,4-dichloropyrimidine, 1500 milliliters of dichloromethane, 1000 milliliters of water and 127.2 grams (1.2mol) of sodium carbonate successively in the 5000ml reaction flask, cool to 0 ℃, keep 89.5 g (1.05 mol) of cyclopentylamine was added dropwise at a reaction temperature of 0-5°C, and the reaction was maintained at 0-5°C for 16 hours after addition. After the reaction is complete, remove the water layer, then add 1000 ml of water, adjust the pH value to 6-7 with hydrochloric acid, separate the water layer, and distill the dichloromethane layer under normal pressure to recover the dichloromethane, and the residue is decomposed with 1140 ml of n-hexane. After purification, the solid was collected by filtration and dried to obtain 261.0 g of 5-bromo-2-chloro-N-cyc...
example 2
[0043] Example 2: Synthesis of 6-bromo-2-chloro-8-cyclopentyl-5-methylpyrido[2,3-D]pyrimidin-7(8H)-one
[0044] (1) Preparation of 5-bromo-2-chloro-N-cyclopentamine-4 amine
[0045] Add 228 grams (1.0mol) of 5-bromo-2,4-dichloropyrimidine successively to a 5000ml reaction flask, recover dichloromethane at 1500, 1000 milliliters of water and 48 grams (1.2mol) of sodium hydroxide, cool to 0°C, Keeping the reaction temperature at 0-5°C, add 89.5 g (1.05mol) of cyclopentylamine dropwise, and keep at 0-5°C for 16 hours after the addition. After the reaction is complete, remove the water layer, add 1000 ml of water, adjust the pH value to 6-7 with hydrochloric acid, separate the water layer, and distill the dichloromethane layer under normal pressure, reclaim the dichloromethane, and use 1140ml of dichloromethane for the remainder. The n-hexane was refined, the solid was collected by filtration, and dried to obtain 262.5 g of 5-bromo-2-chloro-N-cyclopentaline-4amine as a white soli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com