Chitosan nano-selenium particles, preparation method thereof and application of chitosan nano-selenium particles in vaccines
A chitosan nano and chitosan technology, applied in the field of vaccine development, can solve the problems of inability to convert functional immunogens, premature molecular degradation, and low risk of infection, so as to improve humoral immunity and cellular immune response, and improve richness and the effect of good antigen loading capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~11
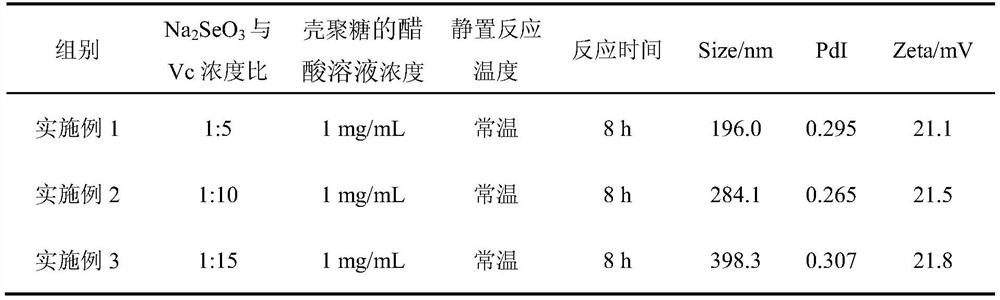
[0035] The preparation of chitosan nano-selenium of the present invention is by changing sodium selenite (Na 2 SeO 3 ), ascorbic acid (Vc), the ratio of chitosan, and conditions such as temperature of reaction, reaction time make the chitosan nano-selenium of different particle diameters, and concrete preparation method is as follows:
[0036] According to the ratio and reaction conditions shown in Tables 1-3, take 2.50 mL of acetic acid solution of chitosan in a 10 mL centrifuge tube, add 40.00 μL of sodium selenite solution and magnetically stir at room temperature for 6 hours, add 200.00 μL of ascorbic acid solution, Add acetic acid solution to make the final volume 5.00mL, mix well and let stand in the dark for 12h at room temperature or under heating conditions. Finally, the chitosan nano-selenium solution that has been reacted is installed in a dialysis bag with a molecular weight cut-off of 10000kD, dialyzed in deionized water at room temperature for 24 hours, and chan...
Embodiment 12~13
[0047] Compared with Example 1 and Example 13, the difference between Example 12 and Example 3 is that the reaction system is increased from 5mL to 250mL, and the rest of the reaction conditions are unchanged, so that Example 12 and Example 3 are obtained. 13 chitosan nano-selenium particles.
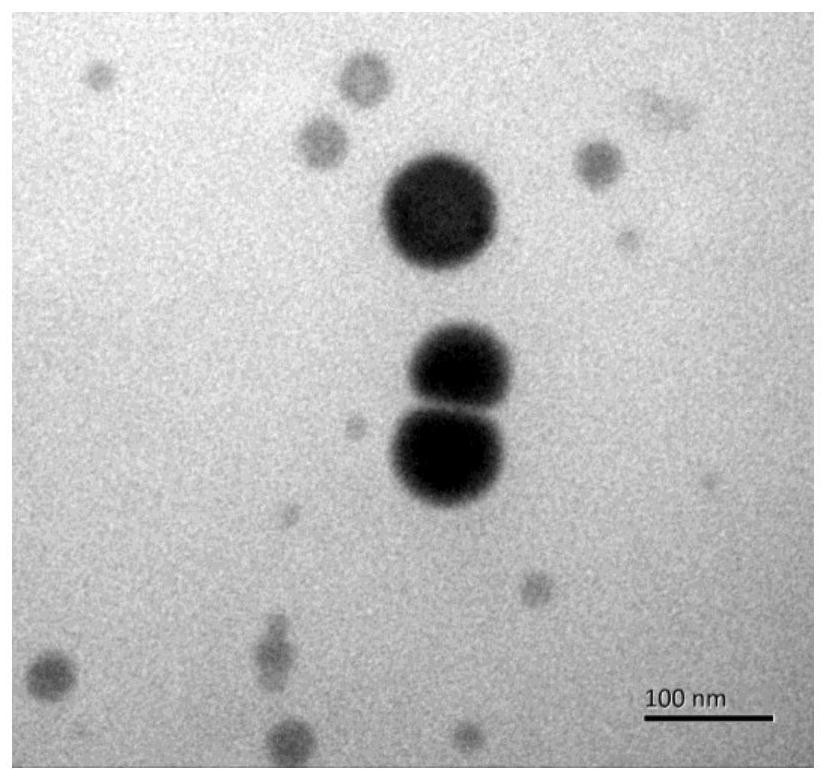
[0048] Such as figure 1 Shown is the transmission electron micrograph of the chitosan nano selenium particle of embodiment 12, from figure 1 It can be seen that the chitosan nano-selenium particles in Example 12 are spherical, with a solid core inside, and the particle diameter is less than 100 nm, about 80 nm.
[0049] The comparison of the measured Size, PdI, and Zeta values of the chitosan nano-selenium particles of Example 12 and Example 13 with the reaction volume of 5 mL is shown in Table 4 below.
[0050] Table 4 reaction system volume is the prepared chitosan nano selenium of 5mL and 250mL
[0051]
[0052] As can be seen from Table 4, the volume of the reaction system h...
Embodiment 14
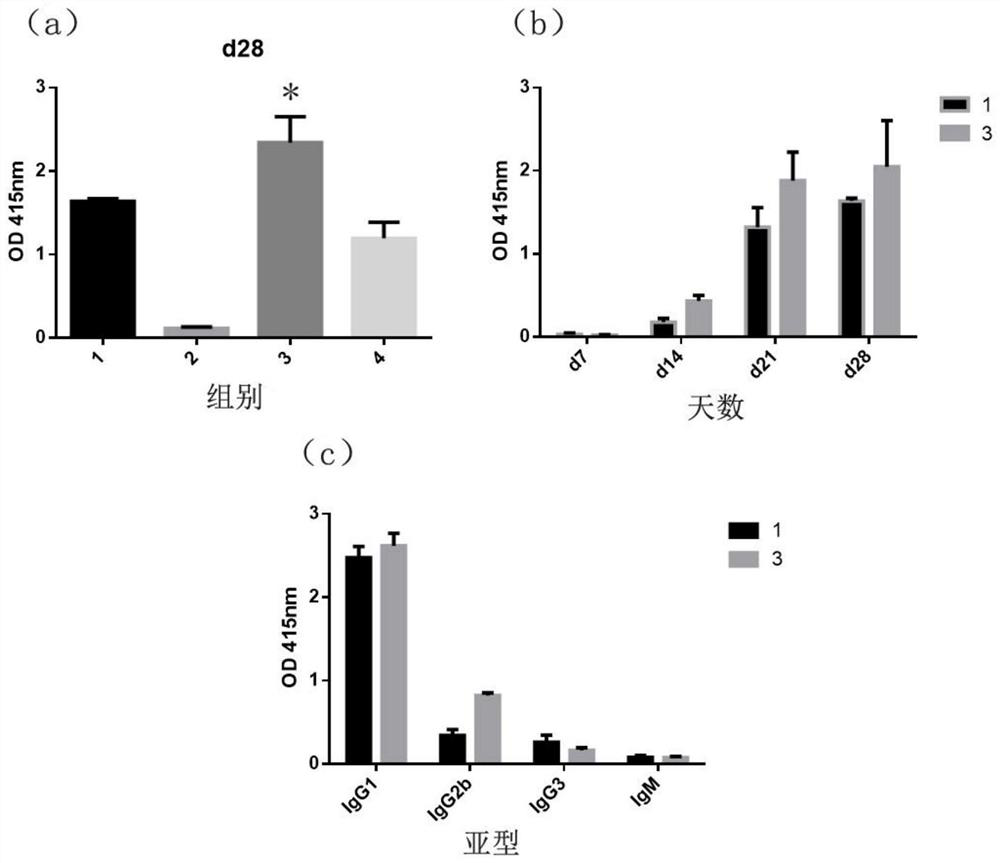
[0068] Because the chitosan nano-selenium particle quality that embodiment 12 and 13 make is the highest, particle uniformity is better, and Zeta potential is higher, therefore, the chitosan nano-selenium particle that makes with embodiment 12 and 13 is sample (respectively Labeled as CS-SeNP-12, CS-SeNP-13) for the preparation and characterization of vaccine samples, mouse immunization experiments.
[0069] 1. Preparation and characterization of vaccine samples
[0070] The first group of vaccine preparation: OVA+chitosan+aluminum adjuvant (vaccine 1), take by weighing 2.8mg chitosan and be dissolved in 0.01mol / L2mL dilute acetic acid solution and then the sodium hydroxide solution of equal concentration and equal volume is neutralized, then use Adjust the pH to 7.4 with high-concentration 1M hydrochloric acid, add OVA, and add aluminum adjuvant at a ratio of 1:1, and mix well.
[0071] Preparation of the second group of vaccines: CS-SeNP-12 (vaccine 2), according to the con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com