Liposome-polymer hybrid nanoparticles for nucleic acid vaccine delivery
A nanoparticle, nucleic acid vaccine technology, applied in liposome delivery, vaccines, DNA/RNA vaccination, etc., can solve the problems of strict protein cold chain requirements, limited application, complex production process of protein and RNA vaccines, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1: Design, Evaluation and Construction of DNA Vaccines
[0057] 1. Epitope screening
[0058] The protein sequences of LTB (1DJR), CFA / I (2HB0), CS6 (4B9J) and IpaB (3U0C) were obtained through the PDB database (https: / / www.rcsb.org / ), and the specific sequences are shown in SEQ ID NO: 3 ., SEQ ID NO: 4., SEQ ID NO: 5. and SEQ ID NO: 6. Shown. ABCpred and Bcepred were used to obtain linear B-cell epitopes, while DiscoTope2.0 and ElliPro were used to predict conformational B-cell epitopes. T cell epitopes are restricted by the major histocompatibility complex (MHC) and are divided into two classes, MHC-I and MHC-II. NetMHCpan4.0 and IEDB were used to obtain MHC-I and MHC-II, respectively, while RANKPEP was used for both MHC types.
[0059] Likewise, CTLPred and PAComplexc were used to obtain suitable CTL epitope candidates. The selected 16 B cell and T cell epitopes (shown in Table 1) have good hydrophilicity, good flexibility, high accessibility and strong a...
Embodiment 2
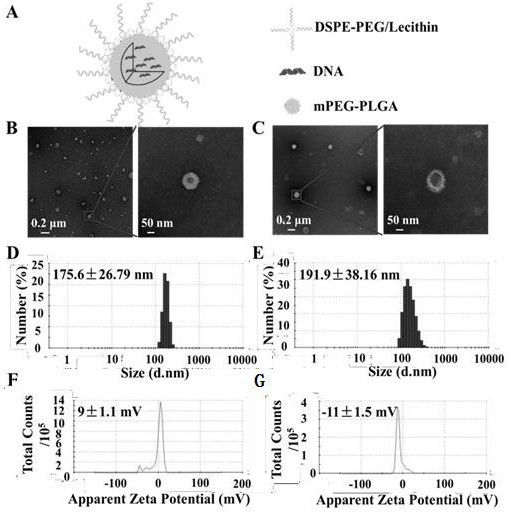
[0069] Example 2: Preparation and Characterization of Liposome-polymer Hybrid Nanoparticles Encapsulating MEG for Nucleic Acid Vaccine Delivery
[0070] 1. Preparation of liposome-polymer hybrid nanoparticles encapsulating MEG
[0071] Dissolve 240 μL of mPEG-PLGA (25 mg / mL) and 50 μg of plasmid DNA (pcDNA3.1, pSFV, pcDNA3.1-MEG, pSFV-MEG, pcDNA3.1-MEG-EGFP or pSFV-EMG-EGFP) in 3 mL of ethyl acetate ester, and the mixture was shaken to form an emulsion to obtain an organic phase. Meanwhile, 84 μL of lecithin (10 mg / mL) and 36 μL of DSPE-PEG2000-Mal (10 mg / mL) (9:1 molar ratio) were dissolved in 9 mL of 4% ethanol solution to obtain an aqueous phase.
[0072] The aqueous phase was preliminarily heated to 65°C, and then the organic phase was added dropwise to the preheated aqueous phase at a rate of 1 mL / min under slow stirring. Afterwards, the mixture was vortexed vigorously for 3 min, then stirred slowly at room temperature for 2 h to evaporate the organic phase. The obtain...
Embodiment 3
[0083] Example 3: Detection of the intracellular expression of MEG
[0084] 1. Detection of transfection efficiency
[0085] The L-02 cells were divided into 2×10 5 Cells / mL were inoculated into 24-well plates, cultured to 80%-90% cell confluence, and the transfection experiment was carried out. One hour before transfection, the medium in the culture plate was discarded, washed 3 times with PBS, and the anti-serum-free medium was replaced. Mix pcDNA3.1-MEG, pSFV-MEG, pcDNA3.1-MEG / LNPs, pSFV-MEG / LNPs, pcDNA3.1-MEG+Lipofectamine, pSFV-MEG+Lipofectamine with basic DMEM medium, add to cells middle. Incubate in an incubator for 4-6 hours, remove particles and liposome complexes, and add pre-warmed complete medium. The culture was continued for 48 h, and the transfection efficiency was detected by flow cytometry, and all experiments were repeated in triplicate.
[0086] like Figure 8 As shown in A, compared with pcDNA3.1-MEG and pSFV-MEG, the transfection efficiency of pcDNA3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com