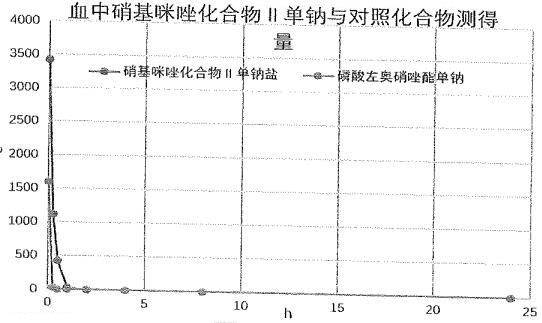
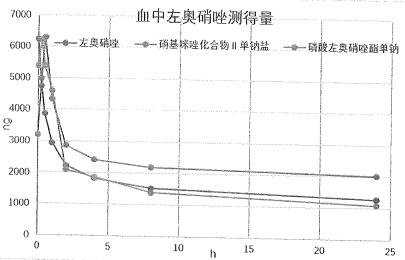
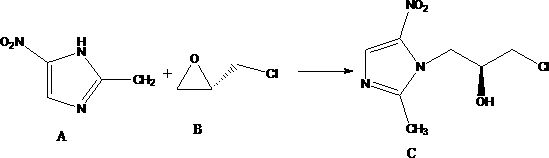
Nitroimidazole derivative, preparation method and application thereof
A technology of nitroimidazoles and nitroimidazoles, which is applied in the field of nitroimidazole derivatives and their preparation, and can solve the problems of increased degree of degradation, unsafety, and high toxicity of degradation products 2-methyl-5-nitroimidazole , to extend the validity period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: the preparation of nitroimidazole compound II
[0041] Add 139.4g of formic acid and 13.3g of concentrated sulfuric acid into the reaction flask, start stirring, then add 40.0g of 2-methyl-5-nitroimidazole. The temperature of the reaction solution was controlled at 0°C to 5°C, and 89.8 g of S-(+)-epichlorohydrin was added dropwise. After the dropwise addition was completed, the temperature was raised to 5° C. to 10° C. and the reaction was stirred for 35 hours, and then the reaction was stopped. Raise the temperature to 85°C-95°C and concentrate under reduced pressure until no liquid flows out from the reflux tube, then stop the concentration. Add 190 g of purified water to the concentrated residue, lower the temperature to 0° C. to 5° C., and then add ammonia water dropwise to adjust the pH to 3 to 4. After the pH is stable, continue stirring for 2 hours. Shake the filter, discard the filter cake, and pump the filtrate into a 100mL reaction flask. The ...
Embodiment 2
[0043] Embodiment 2: the preparation of nitroimidazole compound II
[0044] Add 100ml of acetonitrile, 17g of phosphorus oxychloride and 50g of levonidazole into the reaction flask. Start stirring, heat up to reflux for 4-8 hours, after the reaction, concentrate under reduced pressure until there is no drop, put the residue in a chromatography column, and use dichloromethane:methanol (2:1) for elution, and the elution ends Afterwards, the target eluate was concentrated to 1 / 3 of the total volume, cooled to -5°C to 0°C, and crystallized for 8 to 12 hours. Filtrate, put the filter cake in a blast drying oven, control the temperature at 20°C to 30°C, and dry for 2 to 4 hours to obtain 40.3g of nitroimidazole compound II with a yield of 80.6%.
Embodiment 3
[0045] Embodiment 3: Preparation of nitroimidazole compound II racemate
[0046] Add 100ml of acetonitrile, 17g of phosphorus oxychloride and 50g of ornidazole into the reaction flask. Start stirring, heat up to reflux for 4-8 hours, after the reaction, concentrate under reduced pressure until there is no drop, put the residue in a chromatography column, and use dichloromethane:methanol (2:1) for elution, and the elution ends Afterwards, the target eluate was concentrated to 1 / 3 of the total volume, cooled to -5°C to 0°C, and crystallized for 8 to 12 hours. Filtrate, put the filter cake in a blast drying oven, control the temperature at 20°C to 30°C, and dry for 2 to 4 hours to obtain 43.5 g of racemate of nitroimidazole compound II, with a yield of 87.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com