End-nitrated 3, 4-perylene dicarboxylate compound as well as synthesis method and application thereof
A technology of ester compounds and perylene dicarboxylates, which is applied in the preparation of nitro compounds, chemical instruments and methods, organic chemistry, etc., can solve problems such as less research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] In a second aspect, the present invention provides a method for synthesizing a terminal nitrated 3,4-perylene dicarboxylate compound, comprising the steps of:
[0032] Dissolving 3,4-perylene dicarboxylate compounds in a non-polar organic solvent, adding nitric acid or nitric acid solution dropwise under low temperature conditions, stirring and reacting; the 3,4-perylene dicarboxylate compounds are 3,4-perylene dicarboxylate or 1,6-bis(phenol)-3,4-perylene dicarboxylate compounds;
[0033] After the reaction is completed, the reaction mixture is separated and purified to obtain terminal nitrated 3,4-perylene dicarboxylate compounds.
[0034] In some embodiments, the nitric acid or nitric acid solution is added dropwise at a temperature of 0-20°C.
[0035] For 1,6-bis(phenolic)-3,4-perylenedicarboxylate, when the reaction temperature is controlled at 0-10°C, a mononitration reaction will occur, that is, a nitro group is connected at the 9-position, and the temperature i...
Embodiment 1
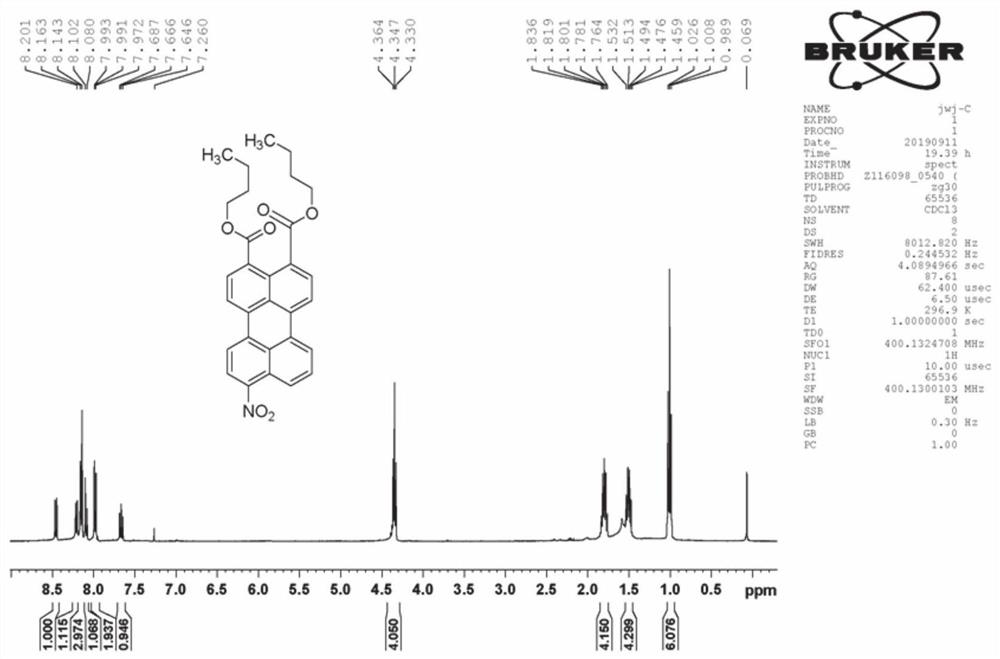
[0062] 200 mg of n-butyl 3,4-perylenedicarboxylate was dissolved in 20 ml of dichloromethane, and 2 ml of 2% fuming nitric acid in dichloromethane was added dropwise at 0° C., followed by TLC until the end of the reaction. The reaction solution was washed 3 times with water, and dried after removing the solvent. The crude product was separated by silica gel column chromatography, and the eluent was dichloromethane. Obtain 9-nitro-3,4-perylene dicarboxylic acid n-butyl ester (product A structure sees attached figure 1 Shown) 144 mg, yield 65%.
Embodiment 2
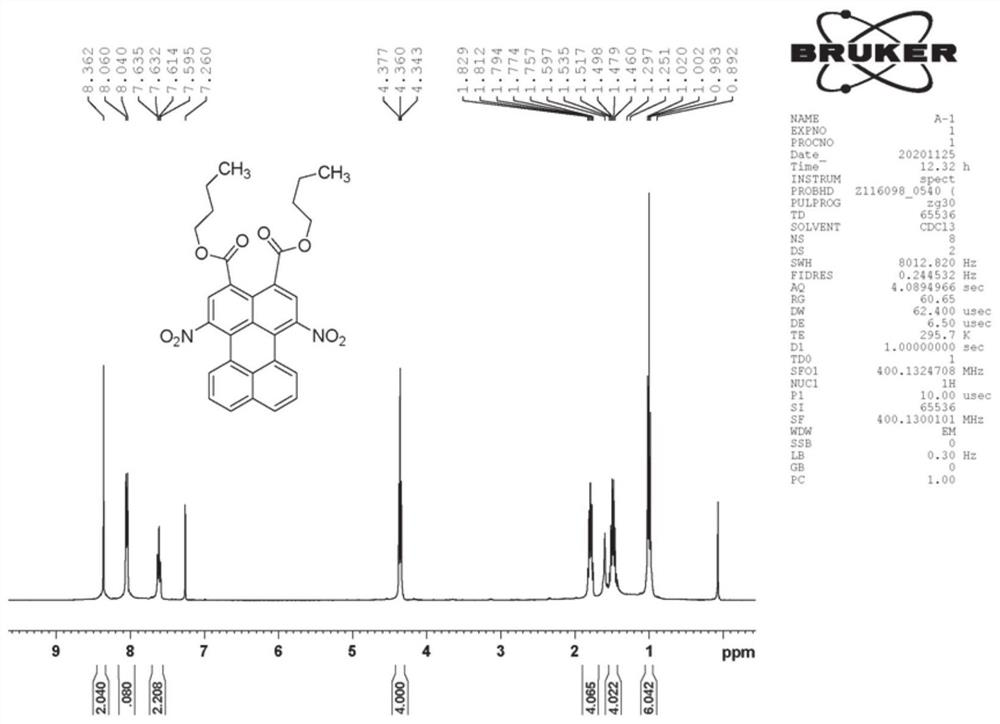
[0064] 200 mg of n-butyl 3,4-perylenedicarboxylate was dissolved in 20 ml of dichloromethane, and 1.5 ml of 5% fuming nitric acid in dichloromethane was added dropwise at room temperature, followed by TLC until the reaction was completed. The reaction solution was washed 3 times with water, and dried after removing the solvent. The crude product was separated by silica gel column chromatography, and the eluent was dichloromethane. Obtain 1,6-dinitro-3,4-perylene dicarboxylate n-butyl (product B-1 structure sees attached figure 2 , 3 Shown) 127 mg, yield 53%.
[0065] Take 120 mg of n-butyl 1,6-dinitro-3,4-perylenedicarboxylate and dissolve it in 8 ml of NMP, add 300 mg of p-tert-butylphenol, 90 mg of anhydrous potassium carbonate, catalytic amount KI, argon Under air protection, the reaction was stirred at 80°C for 12 hours. After the reaction, the reaction liquid was poured into 200 ml of 10% hydrochloric acid aqueous solution, suction filtered, and vacuum-dried to obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com