Cyclodextrin drug inclusion compound as well as preparation method and application thereof
A cyclodextrin and drug technology, applied in the field of cyclodextrin drug inclusion compound and its preparation, can solve the problems of low permeability of intestinal epithelial cells and low bioavailability of insulin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
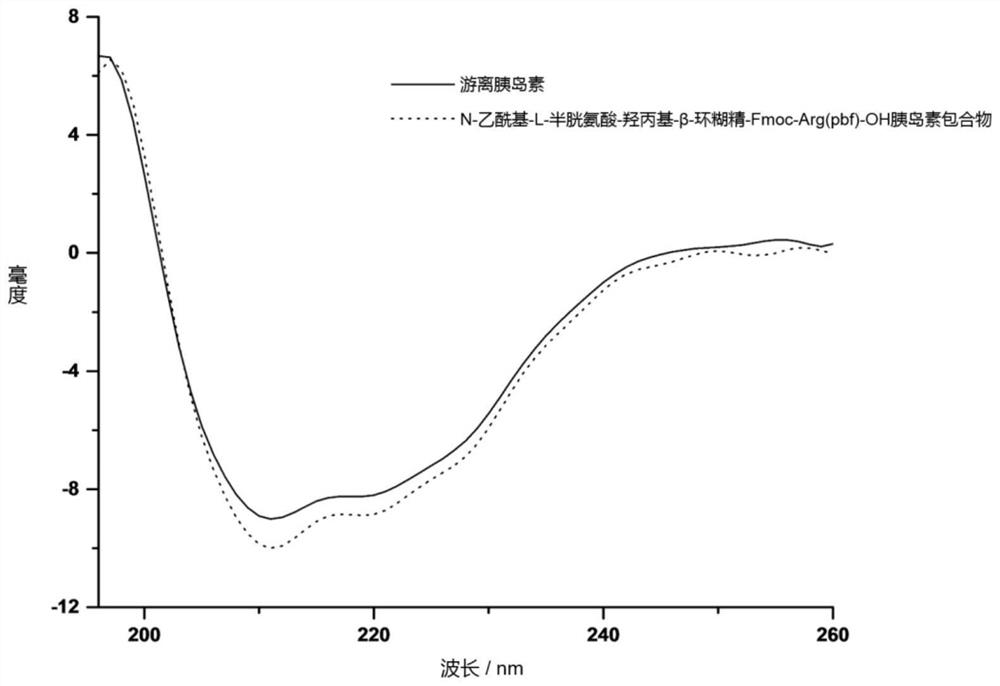
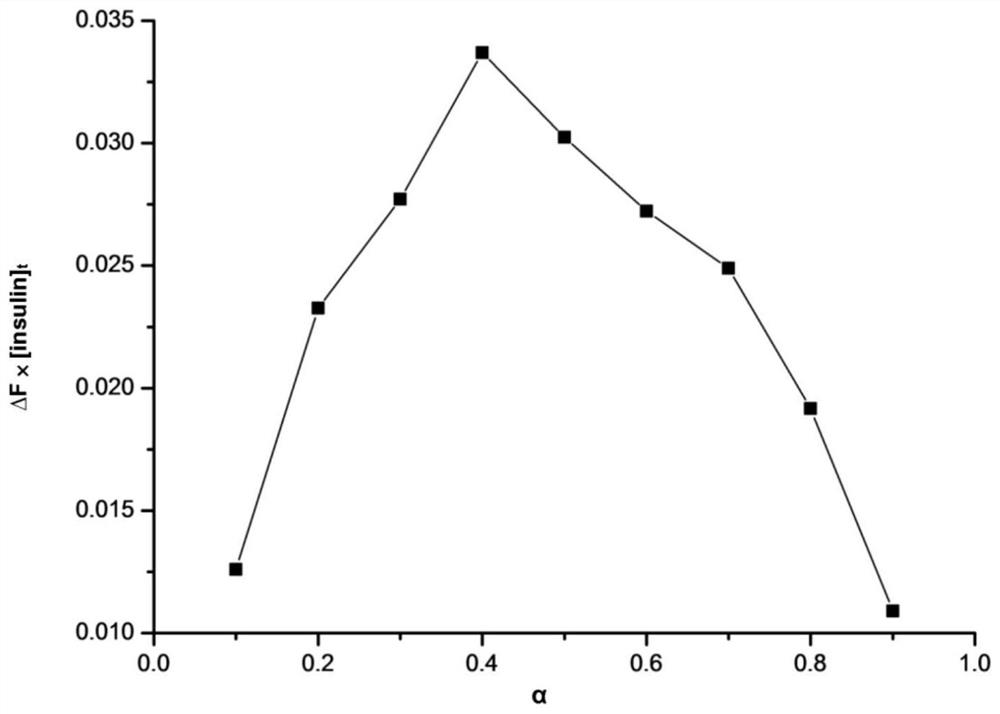
[0025] In order to solve the above problems, the present invention provides a cyclodextrin drug inclusion complex that can not only prevent insulin from being destroyed in the digestive tract, but also prolong the residence time of insulin, thereby improving the absorption efficiency of insulin and its preparation method.
[0026] Specifically, the present invention provides a cyclodextrin graft copolymer for inclusion of insulin in the first aspect, wherein, the cyclodextrin graft copolymer comprises: cyclodextrin as a parent skeleton and graft Sulfhydryl-containing amino acids and guanidine-containing amino acids branched on the parent backbone. The cyclodextrin graft copolymer can be used as a host molecule to form a host-guest inclusion complex with insulin as a guest molecule.
[0027] In some preferred embodiments, the cyclodextrins include but are not limited to α-cyclodextrin, β-cyclodextrin, γ-cyclodextrin, hydroxypropyl-β-cyclodextrin, hydroxypropyl-γ - Cyclodextrin...
Embodiment 1
[0056] Synthesis of Product A
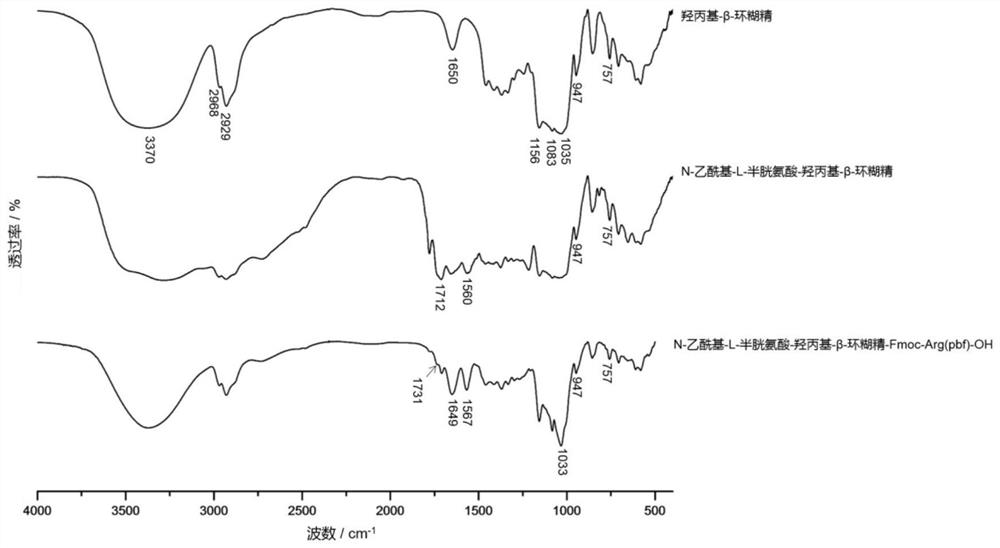
[0057] Weigh 880mg EDC, 530mg NHS, and 150mg thiol-containing amino acids respectively. In a round-bottomed flask, dissolve the mercapto-containing amino acid in the first reaction solvent, then add EDC and NHS and mix well, and stir and activate at room temperature for 3 h. After the reaction was completed, 257 mg of cyclodextrin was added, and stirring was continued at room temperature for 24 h. After the reaction, the mixture was freeze-dried, and acetone was added to sonicate for 0.5 h, and the acetone was removed by filtration to obtain product A.
[0058] When synthesizing product A, the amino acid containing thiol used is N-acetyl-L-cysteine, and the first reaction solvent is distilled water; the cyclodextrin is hydroxypropyl-β-cyclodextrin with a molecular weight of 1399; The molar ratio of amino acid containing thiol to the first catalyst is 1:5; the molar ratio of EDC to NHS is 1:1; the molar ratio of amino acid containing mercapto t...
Embodiment 2
[0066] Synthesis of Product A
[0067] Weigh 880mg EDC, 530mg NHS, and 150mg thiol-containing amino acids respectively. In a round-bottomed flask, dissolve the mercapto-containing amino acid in the first reaction solvent, then add EDC and NHS and mix well, and stir and activate at room temperature for 3 h. After the reaction was completed, 290 mg of cyclodextrin was added, and stirring was continued at room temperature for 24 h. After the reaction, the mixture was freeze-dried, and acetone was added to sonicate for 0.5 h, and the acetone was removed by filtration to obtain product A.
[0068] When synthesizing product A, the amino acid containing thiol used is N-acetyl-L-cysteine, and the first reaction solvent is distilled water; the cyclodextrin is hydroxypropyl-γ-cyclodextrin with a molecular weight of 1580; The molar ratio of mercapto amino acid to the first catalyst is 1:5; the molar ratio of EDC to NHS is 1:1; and the molar ratio of amino acid containing mercapto to cy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com