Antibody or antigen binding fragment thereof for novel coronavirus nucleocapsid protein and application of antibody or antigen binding fragment thereof
A technology of nucleocapsid protein and coronavirus, which is applied in the direction of antiviral immunoglobulin, virus/bacteriophage, virus, etc., and can solve the problems of high technical requirements, prone to false negatives, high false positive rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Example 1 The acquisition of hybridoma cell lines and the preparation of monoclonal antibodies
[0106] This embodiment is used to obtain hybridoma cell lines and prepare monoclonal antibodies. The specific steps are as follows:
[0107] 1.1 Animal immunity
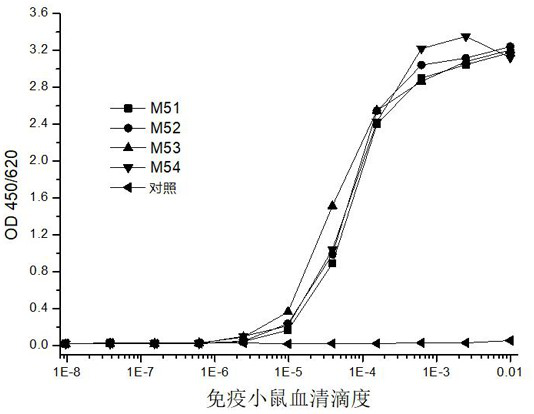
[0108] After fully emulsifying the SARS-CoV-2 N protein antigen (Sino Biological, 40588-V08B) with complete Freund's adjuvant, immunize male Balb / C mice (Shanghai Slack Experimental Animal Co., Ltd.) by multi-point immunization, 50 μg / monkey, the immunization cycle is once every three weeks;
[0109] On the 10th day after the third immunization, blood was collected from the eye socket, and the serum antibody titer was tested according to the indirect ELISA method to monitor the degree of immune response of the mice.
[0110] 1.2 Determination of immune serum titer
[0111] Establish an indirect ELISA method to measure the titer of immune serum: Coat polystyrene micro-96-well plates with 0.1 μg / mL recombinant SAR...
Embodiment 2
[0134] Example 2 Double Antibody Sandwich ELISA Screening for the Best Matching Monoclonal Antibody
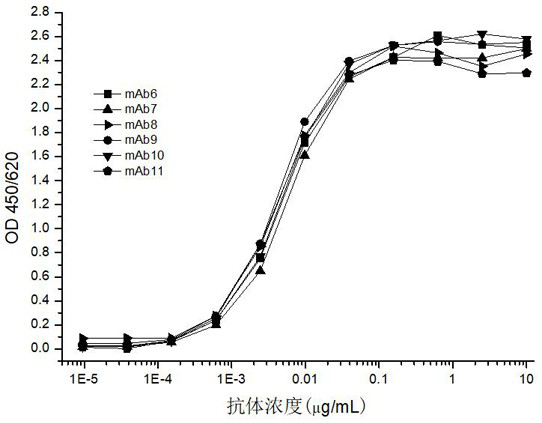
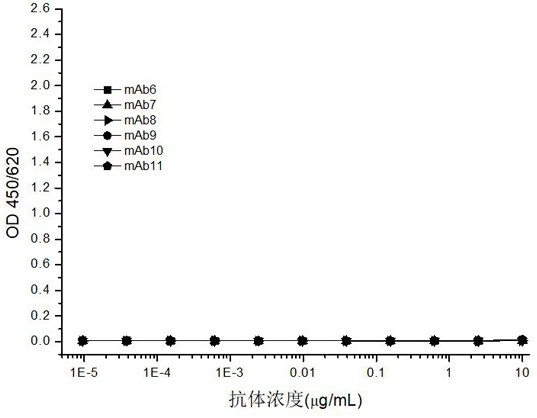
[0135] The antibody produced by monoclonal hybridoma cells only targets one epitope of the antigen, and the "double-antibody sandwich method" is used to screen the best paired solid-phase antibody and labeled antibody.
[0136]A 6×6 matrix was used for pairwise screening of antibodies, and the above-mentioned 6 monoclonal antibodies were coated as capture antibodies, which were combined with 6 biotin-labeled monoclonal antibodies Bio-mAb6, Bio-mAb7, Bio-mAb8, Bio-mAb9, Bio-mAb10, and Bio-mAb11 were paired for rapid screening of captured and labeled mAb pairs in a sandwich ELISA.
[0137] By comparing the detection sensitivity, it was shown that when the monoclonal antibody mAb7 or mAb11 was used as the capture antibody and paired with Bio-mAb6, Bio-mAb8, Bio-mAb9 and Bio-mAb10, respectively, they could generate stronger signals.
Embodiment 3
[0138] Example 3 Functional Identification of Anti-N Protein Monoclonal Antibody
[0139] 3.1 Determination of monoclonal antibody affinity constant
[0140] The binding affinity constants of the purified mouse monoclonal antibodies mAb6, mAb7, mAb8, mAb9 and the N protein of the novel coronavirus were determined by biofilm interferometry (BLI). The ForteBio Octet RED&QK platform of PALL Company was used for determination, and the method was referred to the instruction manual of the platform.
[0141] First, the biotinylated SARS-CoV-2 N protein was immobilized on the surface of the SA sensor, and the above-mentioned monoclonal antibody against the SARS-CoV-2 N protein was used as the analyte. Process the data, and use the analysis software to fit the model of 1:1 binding. The fitted data basically overlaps with the experimental data, and the association and dissociation rate constants are obtained. K a and K d ,use K d remove K a Get the equilibrium dissociation cons...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com