Camptothecin-polycaprolactone coupling prodrug, preparation method thereof, preparation, and preparation method and application of preparation
A technology of polycaprolactone and camptothecin, which is applied in preparation and its preparation, and in the field of camptothecin-polycaprolactone coupling prodrugs, can solve the problems of patients with drug diarrhea and severe gastrointestinal side effects, and achieve Good biocompatibility, good clinical application prospects, and low immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
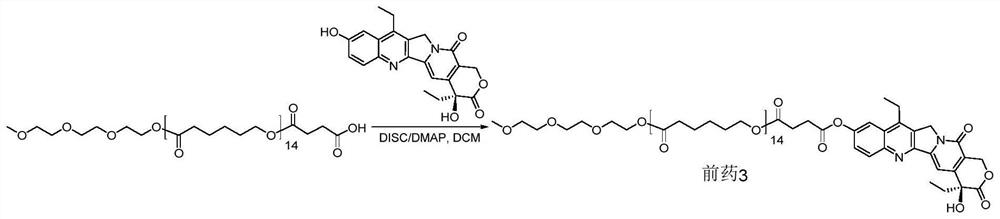
[0073] mPCL 14 -C 2 -COOH is prepared as follows: Synthesis
[0074] Step 1: mPCL 14 synthesis
[0075]
[0076] To a solution of triethylene glycol monomethyl ether (0.931 g, 1.0 eqv.) and ε-caprolactone (9.069 g, 14.0 eqv.) in anhydrous toluene (50 ml) was added a catalytic amount of Sn(Oct) 2 . The mixture was stirred overnight at 150° C. for ring-opening reaction. After the reaction was completed, the solvent was evaporated under reduced pressure, and the residue was dissolved in DCM and precipitated with cold ether. Filtrate under reduced pressure, and dry the solid in vacuum to obtain a white powder hydroxyl-terminated oligomer 14 (mPCL 14 ) (8.821 g, 88%).
[0077] Step 2: mPCL 14 -C 2 -COOH synthesis
[0078]
[0079] To a solution of hydroxyl terminated oligomer 14 (8.021 g, 1.0 eqv.) and succinic anhydride (2.279 g, 5.0 eqv.) in dry DCM (30 ml) was added dry pyridine (1.440 g, 4.0 eqv.). The mixture was heated to reflux for five days, followed by 5% ...
Embodiment 1
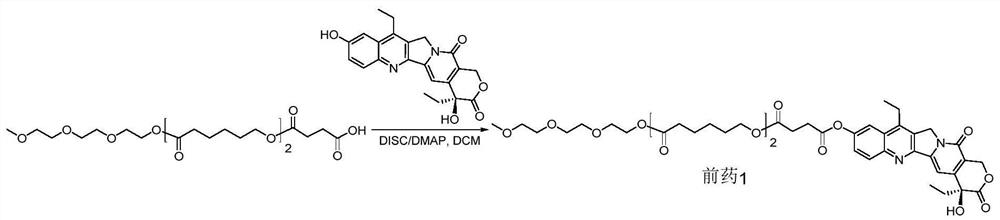
[0081] Example 1 mPCL 2 -C 2 - Synthesis of pSN38 coupled prodrug 1, as figure 1 Shown:
[0082] Add 7-ethyl-10-hydroxycamptothecin (SN38, 200.0mg, 0.5097mmol), mPCL in sequence to a 100mL round bottom flask equipped with a spherical condenser 2 -C 2 -COOH (250.9mg, 0.5097mmol, structure as figure 1 shown), and DMAP (74.8mg, 0.6116mmol), dissolved in 10mL of anhydrous dichloromethane, and then quickly dropwise added DISC (N,N'-diisopropylcarbodiimide) (193.1mg, 1.5291mmol) . Stir at 46°C overnight, and observe the reaction by thin-layer chromatography (developing solvent: DCM:MeOH=15:1). When the reaction was basically completed, the reaction liquid was cooled, and then washed with distilled water, 5% citric acid, saturated sodium carbonate, and saturated saline respectively. The organic layer was dried over anhydrous sodium sulfate, and filtered after complete drying. The filtrate was rotary evaporated to remove the solvent. Separation and purification by column chro...
Embodiment 2
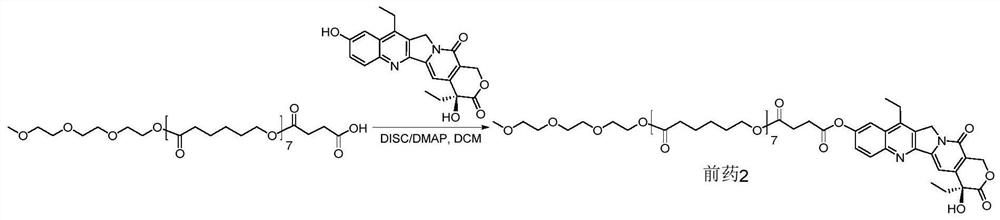
[0083] Example 2 mPCL 7 -C 2 - Synthesis of pSN38 coupled prodrug 2, as figure 1 Shown:
[0084]Add 7-ethyl-10-hydroxycamptothecin (SN38, 300.0mg, 0.7645mmol), mPoCl 7 -C 2 -COOH (812.1mg, 0.7645mmol) and DMAP (112.1mg, 0.9174mmol) were dissolved in 10mL of anhydrous dichloromethane, and DISC (289.4mg, 2.2936mmol) was quickly added dropwise. Stir at 46°C overnight, and observe the reaction by thin-layer chromatography (developing solvent: DCM:MeOH=15:1). When the reaction was basically completed, the reaction liquid was cooled, and then washed with distilled water, 5% citric acid, saturated sodium carbonate, and saturated saline respectively. The organic layer was dried over anhydrous sodium sulfate, and filtered after complete drying. The filtrate was rotary evaporated to remove the solvent. Separation and purification by column chromatography (DCM:MeOH=60:1) to obtain the final product 2 (corresponding figure 2 The structure of n=7, 683.9 mg, yield 61.5%)
[0085] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com