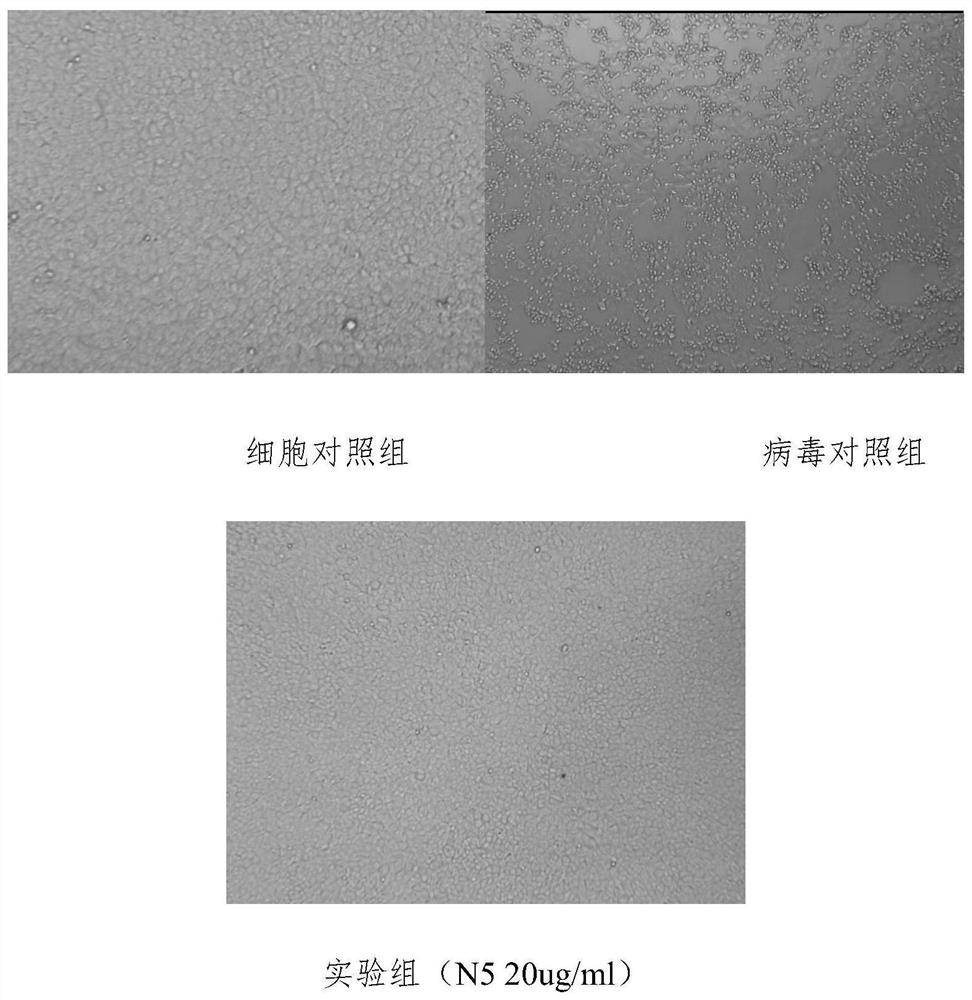
Monoclonal antibody for neutralizing novel coronavirus infection
A coronavirus and antibody technology, applied in antiviral agents, antiviral immunoglobulins, antibodies, etc., can solve problems such as no specific treatment methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1. Preparation of monoclonal antibody N5 and humanized human-mouse chimeric antibody ch-N5
[0045] 1. Preparation of monoclonal antibody N5
[0046] 1. Discovery of monoclonal antibody N5
[0047] The S protein (100ug / ml) of the SARS-CoV-2 virus was emulsified with an equal volume of Freund's complete adjuvant as an immunogen, and the female Balb / C healthy mice (purchased from military Experimental Animal Center, Academy of Medical Sciences), the injection dose was 0.4 mL of immunogen per mouse. Female Balb-c mice were immunized three times with an interval of 2 weeks between each time. From the second booster immunization, on the 3rd day after each immunization, blood was collected from the orbit of the mouse to measure the antibody titer, and the mouse with the best serum titer was selected, and the spleen of the mouse was dissected to prepare a spleen cell suspension. Cultured in complete DMEM medium for 5 days in an incubator. Hybridoma cells secreting ...
Embodiment 2
[0078] Example 2. Application of monoclonal antibody N5 and humanized human-mouse chimeric antibody ch-N5
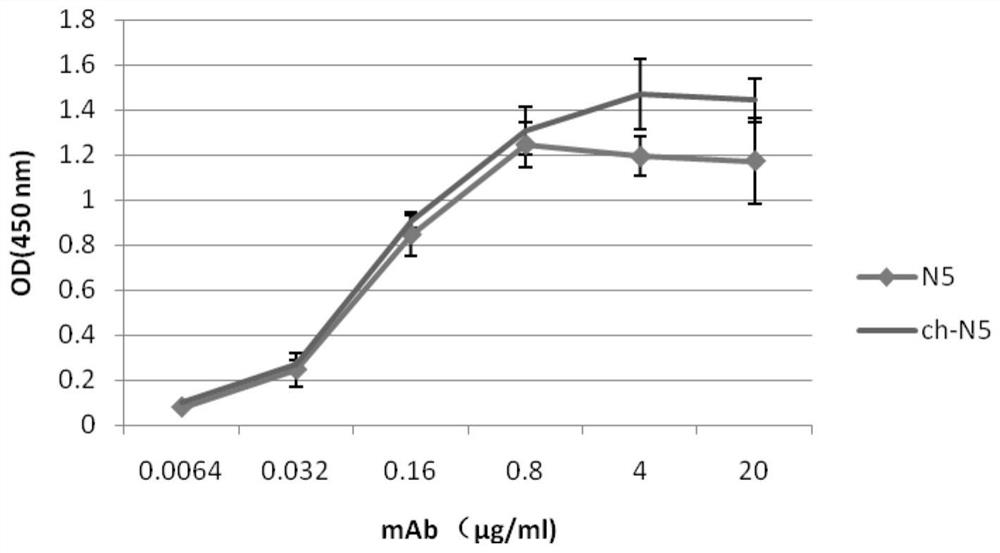
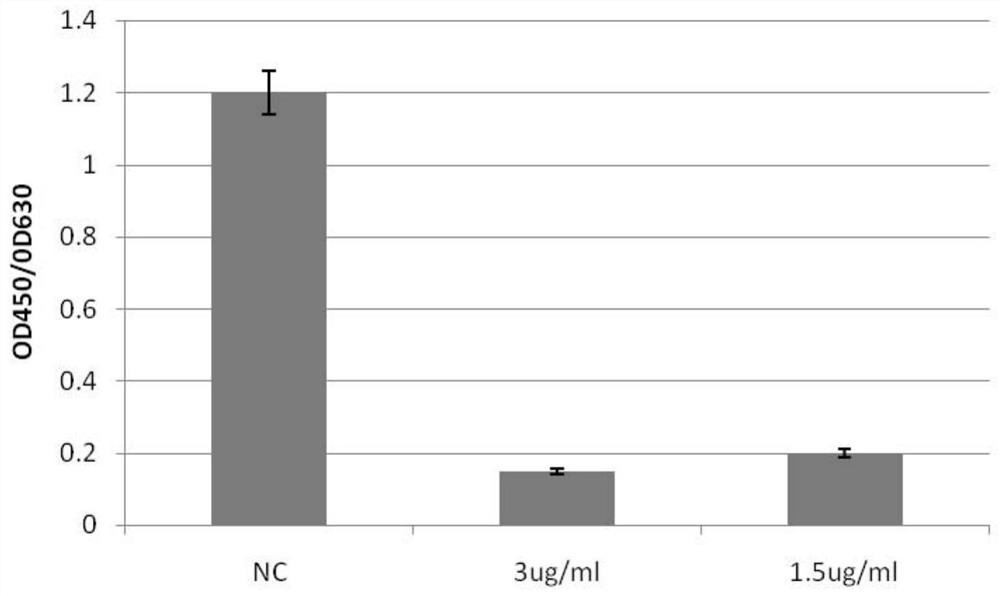
[0079] 1. Monoclonal antibody N5 antibody specifically binds to the RBD region of the S protein of the 2019-nCoV novel coronavirus
[0080] The ELISA method is used to detect the binding activity of the monoclonal antibody N5 antibody to the S protein of the new coronavirus, and the operation steps are as follows:
[0081] 1. Coating: Dilute the recombinantly expressed novel coronavirus S-RBD protein (product of Beijing Boaolong Immunology Technology Co., Ltd., BD-VP1488) to 5 μg / ml, and coat 100 μl / well of an ELISA microwell plate, wherein, The formulation of the test solution is: sodium carbonate / sodium bicarbonate buffer solution (0.1M) with pH 9.6. overnight at 4°C.
[0082] 2. Blocking: wash once with washing solution (recipe: phosphate buffered saline solution containing Tween-20 with a volume percentage of 0.5‰, referred to as PBST washing solution), and then us...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com