Mesenchymal stem cell serum-free medium and application thereof
A technology of serum-free medium and mesenchymal stem cells, which is applied in the field of serum-free medium for mesenchymal stem cells, can solve the problem of unrepeatable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] This embodiment provides a serum-free medium for mesenchymal stem cells, each 1L of the medium consists of the following components:
[0072] IMDM 17.662g, L-alanyl-glutamine 2mM, chemical lipid 0.6v / v%, cholesterol 15mg, insulin 12.5mg, transferrin 25mg, recombinant human albumin 2g, hydrocortisone 500μg, dexamethasone 4μg, progesterone 5.66μg, putrescine 9mg, ascorbic acid 100mg, β-mercaptoethanol 75μM, soybean lecithin 10mg, zinc sulfate heptahydrate 2.5mg, lipoic acid 0.2mg, N-Acetyl-L-Cysteine 1mM, reduced glutathione Glycine 2mg, Taurine 5mg, Y-27632 5μM, Ethanolamine 2mg, b-FGF 20μg, EGF 20μg, PDGF-BB 10μg, IGF-1 15μg, TGF-β3 2μg, HGF 10μg, CTGF 2μg and VEGF 15μg; The amount is water for cell culture.
[0073] This embodiment further provides a method for configuring the above-mentioned serum-free medium for mesenchymal stem cells:
[0074] Dissolve IMDM powder in cell culture water, stir well, filter with 0.22um filter membrane, add recombinant human insulin...
Embodiment 2
[0076] This embodiment provides a serum-free medium for mesenchymal stem cells, each 1L of the medium consists of the following components:
[0077] IMDM 17.662g, L-alanyl-glutamine 2mM, chemical lipid 0.1v / v%, cholesterol 5mg, insulin 8mg, transferrin 10mg, recombinant human albumin 1g, hydrocortisone 0.1mg, dexamethasone 4μg, progesterone 1μg, putrescine 5mg, ascorbic acid 25mg, β-mercaptoethanol 50μM, soybean lecithin 2mg, zinc sulfate heptahydrate 1.25mg, lipoic acid 0.1mg, N-Acetyl-L-Cysteine 0.2mM, reduced glutathione Glycine 1mg, Taurine 2mg, Y-27632 2μM, Ethanolamine 1mg, b-FGF 5μg, EGF 5μg, PDGF-BB 2μg, IGF-1 10μg, TGF-β3 1μg, HGF 2μg, CTGF 1μg and VEGF 5μg; The amount is water for cell culture.
[0078] Its configuration method is the same as embodiment 1.
Embodiment 3
[0080] This embodiment provides a serum-free medium for mesenchymal stem cells, each 1L of the medium consists of the following components:
[0081] IMDM 17.662g, L-alanyl-glutamine 2mM, chemical lipid 1v / v%, cholesterol 30mg, insulin 25mg, transferrin 30mg, recombinant human albumin 5g, hydrocortisone 2mg, dexamethasone 20μg, Progesterone 10μg, putrescine 15mg, ascorbic acid 200mg, β-mercaptoethanol 100μM, soybean lecithin 20mg, zinc sulfate heptahydrate 2.5mg, lipoic acid 0.5mg, N-Acetyl-L-Cysteine 2mM, reduced glutathione 5mg , taurine 10mg, Y-27632 10μM, ethanolamine 5mg, b-FGF 40μg, EGF 50μg, PDGF-BB 20μg, IGF-1 40μg, TGF-β3 5μg, HGF 10μg, CTGF 5μg and VEGF 20μg; the balance was cells Cultivate water.
[0082] Its configuration method is the same as embodiment 1.
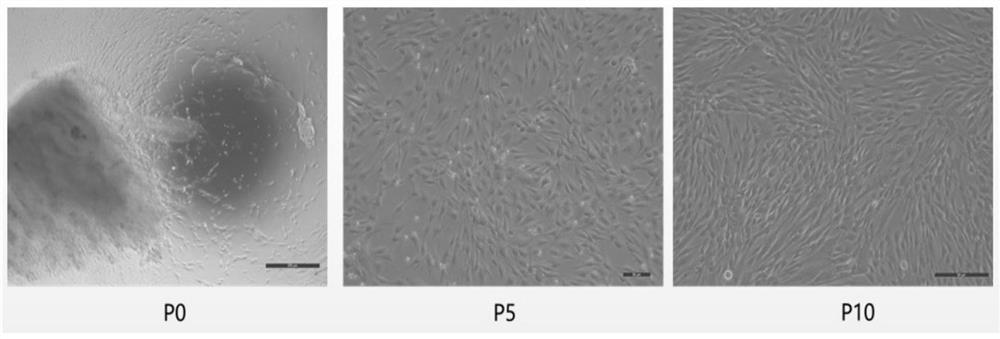
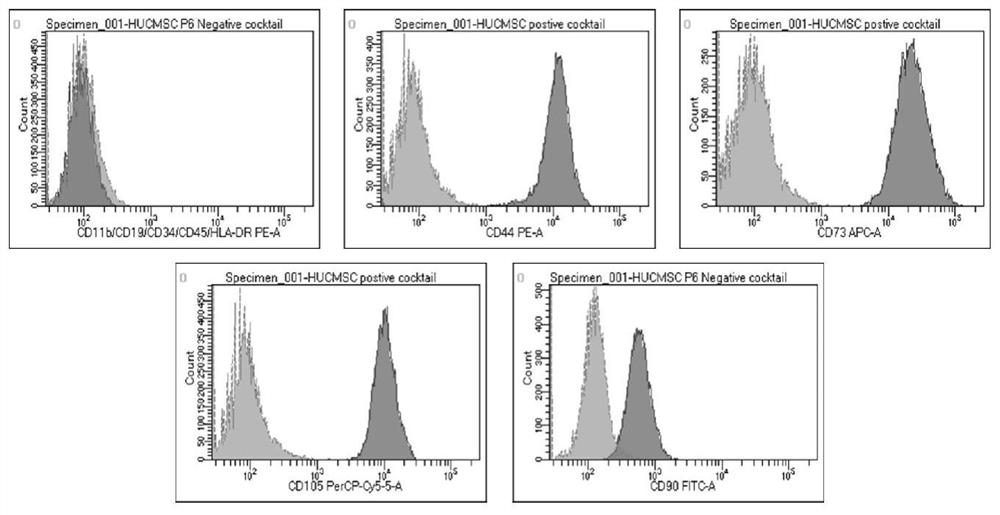
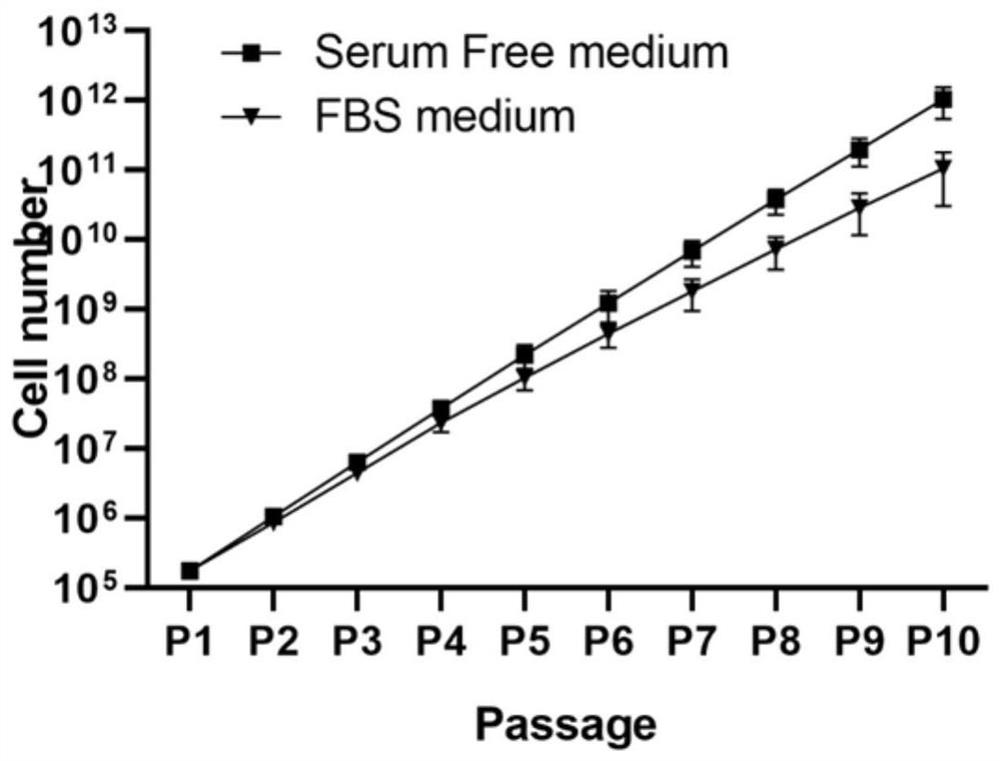
[0083] The same strain of P1 generation human umbilical cord mesenchymal stem cells was taken for testing, and three groups of repeated experiments were carried out in each of Examples 1-3, and the inoculat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com