CHO cell serum-free protein-free culture medium and application thereof
A protein-free medium and serum-free technology, applied in the field of biomedicine, can solve problems such as disjointed R&D or production processes, complicated equipment, and affecting the development of perfusion culture processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
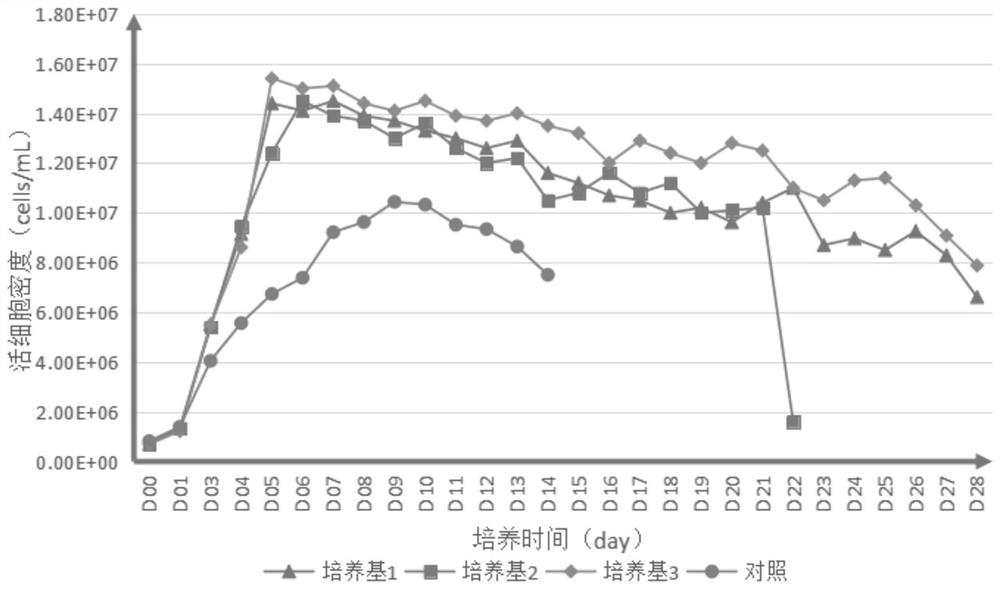
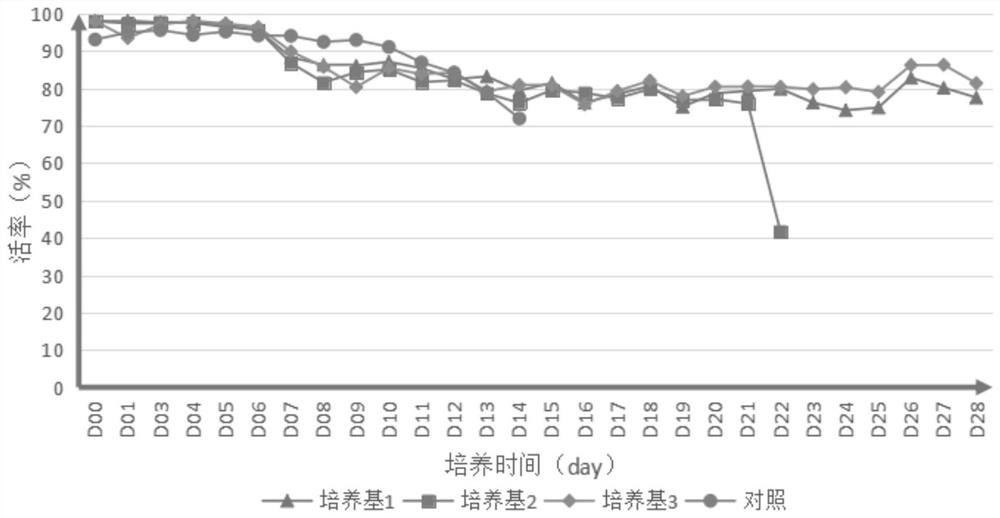
[0098] 1. Prepare the medium for each experiment according to the following material concentration: Add 1 IL of each component into 800ml ultrapure water, stir at room temperature for 30 minutes, and adjust the pH to 6.9-7.3 with 1M freshly prepared sodium hydroxide solution or hydrochloric acid. Filter through a 0.22 μm sterile membrane and store at 4°C for long-term use.
[0099] The composition of amino acids is:
[0100] L-Arginine 150-800mg / L
[0101] L-Aspartic Acid 100-550mg / L
[0102] L-Asparagine 145-500mg / L
[0103] L-cysteine 105-350mg / L
[0104] L-Glycine 20-110mg / L
[0105] L-glutamic acid 50-180mg / L
[0106] L-histidine 145-435mg / L
[0107] L-Isoleucine 225-550mg / L
[0108] L-Leucine 160-480mg / L
[0109] L-Lysine 270-700mg / L
[0110] L-Methionine 65-145mg / L
[0111] L-phenylalanine 130-460m / L
[0112] L-proline 135-470mg / L
[0113] L-serine 200-500mg / L
[0114] L-Threonine 50-300mg / L
[0115] L-Tryptophan 160-420mg / L
[0116] L-tyrosine 180-540mg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com