Preparation method and application of antigen and adjuvant co-delivery nano vaccine based on protamine as carrier
A nano-vaccine, protamine technology, applied in the field of immunology, can solve the problems of low antigen delivery efficiency, affecting the effect of antigen immunotherapy, poor immunogenicity, etc., achieving effective immunotherapy effect, realizing immunotherapy effect, and convenient preparation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Preparation of Nanovaccine
[0062] Dissolving PRT in PBS buffer solution, the concentration of PRT is 0.1-20 mg / mL to form a PRT solution. Ovalbumin (OVA, as a model antigen) was dissolved in a PBS buffer solution with a concentration of 0.1-5 mg / mL to form an OVA solution. CpG is dissolved in PBS buffer solution, and the concentration of CpG is 0.1-5 mg / mL to form a CpG solution. The PRT solution, the OVA solution and the CpG solution were mixed, vortexed for 20 seconds, and then left to stand at room temperature for 25 minutes to obtain the nanovaccine PRT / CpG / OVA.
Embodiment 2
[0064] Nano vaccine particle size distribution
[0065] The nano-vaccine prepared in Example 1 was studied for particle size distribution. The average particle size, Zeta potential and particle size dispersion index (PDI) were determined by nanometer particle size and Zeta potential analyzer. Refer to Table 1 for the results.
[0066] Potential, particle size and PDI test result of table 1 embodiment 1
[0067]
Embodiment 3
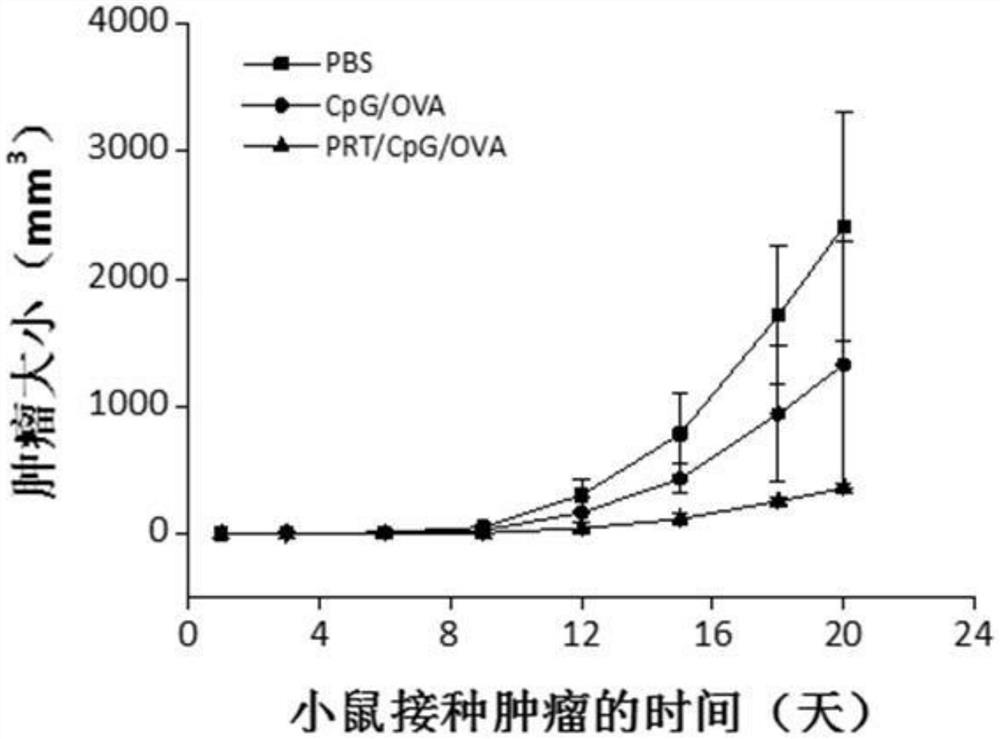
[0069] Effect experiment
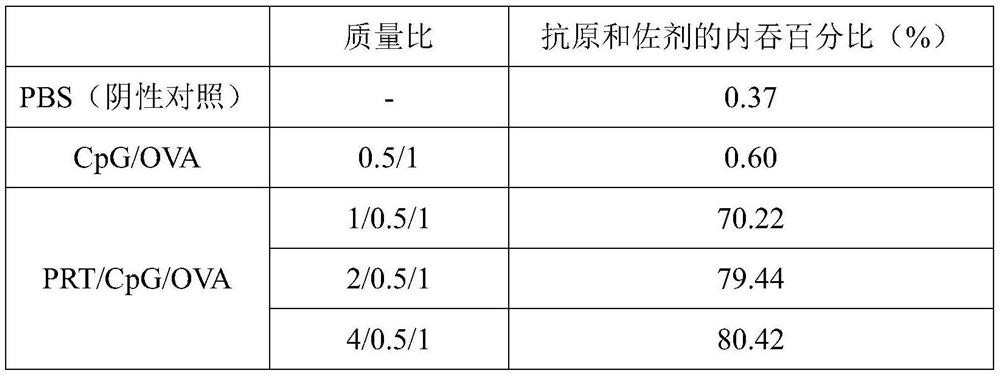
[0070] (1) Endocytosis of nanovaccine in antigen-presenting cells
[0071] Mouse bone marrow-derived dendritic cells (BMDCs) were selected, bone marrow cells were obtained from mice, stimulated and induced in vitro, and differentiated into BMDCs after seven days of culture. After culturing BMDCs for seven days, use a pipette to absorb the medium and gently blow the cells, collect the cell suspension and place it in a 15mL centrifuge tube, centrifuge at 1000rpm for 3min to collect the cells; 6 The density of cells was seeded in 6-well plates and cultured overnight in an incubator containing 5% carbon dioxide by volume at 37°C. Then add sample materials (the samples are: PBS, CpG / OVA-FITC and PRT / CpG / OVA-FITC respectively, the final concentration of OVA added is 5 μg / mL), continue to cultivate for 4 hours, and then use flow cytometry to detect the antigen and endocytosis of adjuvants.
[0072] The above-mentioned OVA-FITC is OVA labeled with fluores...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com