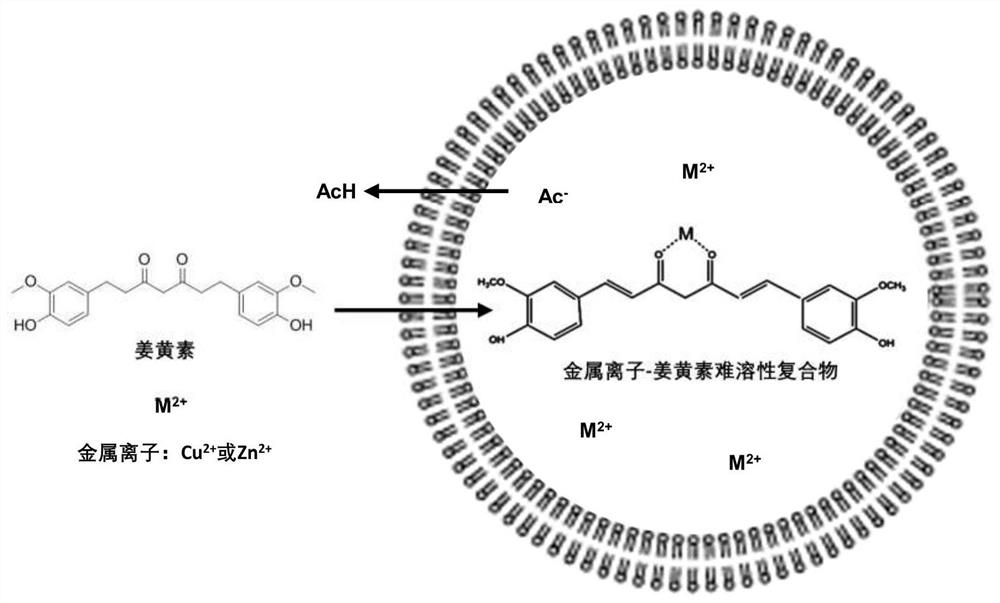
Curcumin active drug-loading liposome and preparation method thereof
A technology of curcumin lipid and curcumin, which is applied in the field of curcumin active drug-loaded liposomes and its preparation, can solve problems such as poor stability, decreased cell activity of drug-loaded preparations, and general drug effects, so as to improve accumulation and benefit The effect of cellular uptake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0065] Inner aqueous phase concentration of embodiment 2 has influence on curcumin liposome particle size and encapsulation efficiency
[0066] According to the method in Example 1 and using copper acetate as the internal water phase, the concentration of the internal water phase was set according to the table below to prepare blank liposomes. The prepared blank liposomes and curcumin were co-incubated at 65°C for 30 minutes according to the drug-to-lipid ratio of 1:10. After the drug loading was completed, the drug loading was terminated in ice bath, and the liposome particle size and encapsulation efficiency were measured. The results were See image 3 .
[0067]
[0068]
[0069] The result shows: along with the increase of internal aqueous phase concentration, curcumin encapsulation efficiency also increases, and when concentration is greater than 200mM, liposome encapsulation efficiency declines to some extent, and particle diameter also increases significantly.
Embodiment 3
[0070] The effect of embodiment 3 drug-lipid ratio on liposome particle size encapsulation efficiency
[0071] Weigh 68.1mg of HSPC, 22.8mg of Chol, and 1.2mg of DSPE-PEG2000, dissolve them in chloroform, remove the organic solvent by rotary evaporation under reduced pressure at 37°C to form a phospholipid film, and hydrate with 200mM copper acetate solution at 65°C for 30min to obtain the crude product The liposome solution passes through 0.4, 0.2, 0.1 μm polycarbonate membranes sequentially in the extrusion device, and the particle size is measured. The metal ions outside the liposomes were removed by Sephadex chromatography column exchange, and the outer water phase was exchanged with HEPES buffer to obtain blank liposomes. Dissolve curcumin in DMSO, prepare 20mg / mL curcumin solution, add 50μL curcumin solution to 1mL 10mg / mL blank liposomes, incubate at 65°C for 30 minutes, stop drug loading in ice bath, and measure particle size. diameter and encapsulation efficiency, th...
Embodiment 4
[0073] The impact of embodiment 4 external water relative curcumin liposome encapsulation efficiency
[0074] Weigh 68.1mg HSPC, 22.8mg Chol, 1.2mg DSPE-PEG2000, dissolve in chloroform, remove the organic solvent by rotary evaporation under reduced pressure at 37°C to form a phospholipid film, and hydrate with 200mM copper acetate or zinc acetate solution at 65°C for 30min , The resulting crude liposome solution passed through 0.4, 0.2, and 0.1 μm polycarbonate membranes in sequence in an extrusion device, and the particle size was measured. Remove liposome external metal ions by Sephadex chromatographic column exchange and exchange the external water phase for HEPES buffer (pH 5.0), HEPES buffer (pH 6.0), HEPES buffer (pH 7.0), HEPES buffer ( pH 8.0), to obtain blank liposomes. Dissolve curcumin in DMSO, prepare 20mg / mL curcumin solution, add 50μL curcumin solution to 1mL 10mg / mL blank liposomes, incubate at 65°C for 30 minutes, stop drug loading in ice bath, and measure par...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com