Medicinal gel, preparation method thereof and application of medicinal gel
A gel and drug technology, applied in the field of drug gel and its preparation, can solve the problems of strong drug resistance, limited dosage, easy relapse, etc., achieve good sustained release effect, reduce release speed, and prevent drug leakage. seepage effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The second aspect of the present invention provides the preparation method of the above-mentioned pharmaceutical gel, the preparation method comprising:
[0031] (1) Mix and swell the poloxamer 407, poloxamer 188, hydroxypropyl cellulose and water, then add the diphenylheptane A hydroxypropyl-β-cyclodextrin under stirring clathrate and glycerol to obtain the first solution;
[0032] (2) mixing the crisborole, ethylparaben and propylene glycol to obtain a second solution;
[0033] (3) Mixing the first solution and the second solution uniformly to obtain the drug gel.
[0034] According to the present invention, preferably, in step (1), the poloxamer 407, poloxamer 188, and hydroxypropyl cellulose are spread on the surface of water, and then left to swell.
[0035] According to the present invention, preferably, the swelling temperature is 4-8° C., and the swelling time is not less than 24 hours.
[0036] The third aspect of the present invention provides the applicati...
Embodiment 1
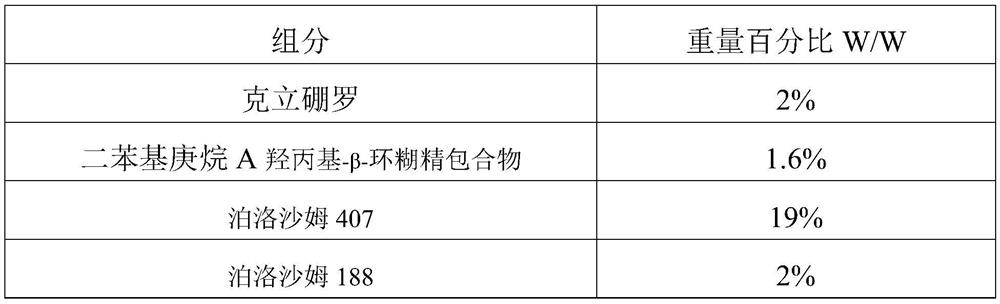
[0039] The present embodiment provides a pharmaceutical gel, the specific formulation is shown in Table 1, and the specific preparation method is as follows:
[0040] (1) Spread poloxamer 407, poloxamer 188, and hydroxypropyl cellulose on the surface of purified water, let it swell at 4°C for 24 hours to obtain a homogeneous solvent, and then add diphenylheptane A hydroxyl Propyl-beta-cyclodextrin inclusion complex and glycerin to obtain the first solution;
[0041] (2) Criborole, ethylparaben and propylene glycol are mixed to obtain a second solution;
[0042] (3) Add the second solution into the first solution under stirring, and stir evenly to obtain the drug gel;
[0043] Wherein, the diphenylheptane A hydroxypropyl-β-cyclodextrin inclusion compound is prepared by the following method:
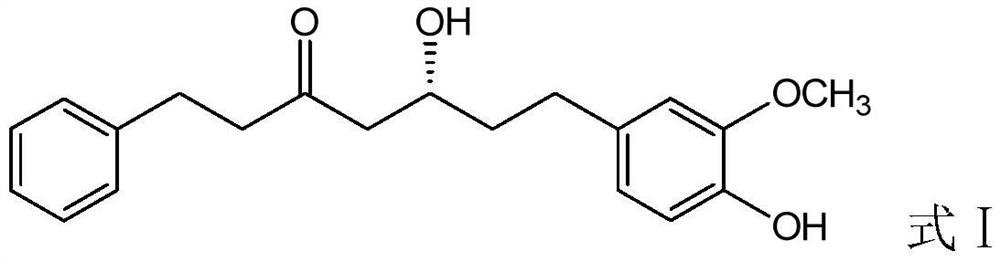
[0044] (1) Weigh 10g of diphenylheptane A, add 10ml of ethanol to dissolve, and obtain diphenylheptane A ethanol solution;
[0045] (2) Mix 50 g of hydroxypropyl-β-cyclodextrin with 200...
Embodiment 2
[0051] This embodiment provides a drug gel, and the specific formula is shown in Table 2;
[0052] The dosage and preparation method of each raw material for preparing diphenylheptane A hydroxypropyl-β-cyclodextrin inclusion complex and the preparation method of the drug gel are the same as in Example 1.
[0053] Table 2
[0054]
[0055]
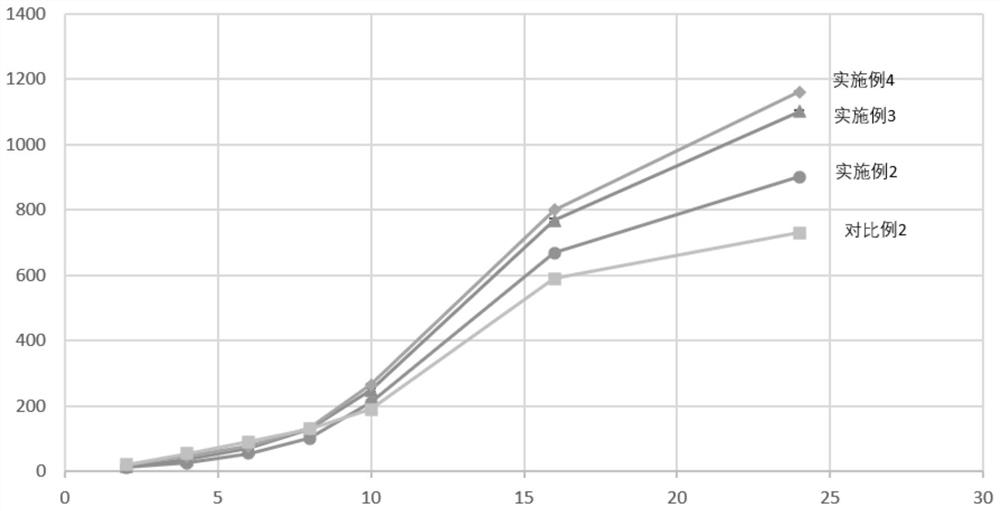
[0056] The mass ratio of crisborole to diphenylheptane A contained in the drug gel prepared in this example is 7.5:1. The phase transition temperature of the pharmaceutical gel prepared in this example is 29°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Phase transition temperature | aaaaa | aaaaa |
| Phase transition temperature | aaaaa | aaaaa |
| Phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com