Compound, display panel and display device
A display panel and compound technology, applied in the field of OLED, can solve problems such as the difficulty in developing doped materials, achieve the effects of widening the exciton recombination region, high glass transition temperature and thermal stability, and reducing intermolecular concentration quenching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
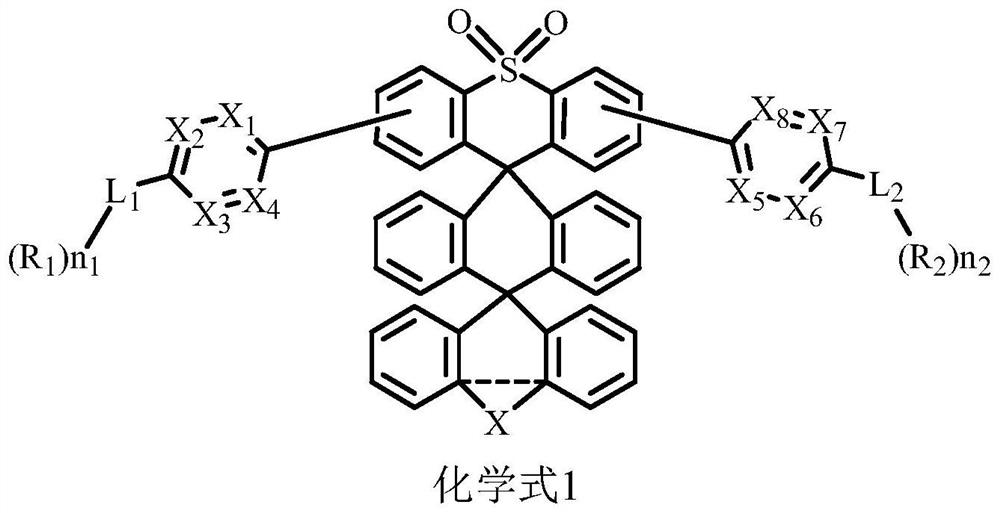
[0025] According to one embodiment of the compound of the present invention, the compound has the structures shown in formula 1-1 to formula 1-6:
[0026]
[0027] According to one embodiment of the compound of the present invention, the compound is selected from the compounds represented by formula 1-1 to formula 1-4.
[0028] Further, the compound is selected from the compounds represented by formula 1-1, formula 1-3 or 1-4. Formulas 1-1, 1-3, 1-4, such connection methods may make it easier to form intramolecular and intermolecular C-H...N bonds, so that the easily broken carbon-nitrogen (C-N) bonds are strengthened, and the bonds of the molecules are dissociated The energy may be improved, making it more stable in the preparation of the device, which is beneficial to improving the life of the device.
[0029] According to one embodiment of the compound of the present invention, R 1 and R 2 each independently selected from any of the following groups:
[0030]
[0...
Embodiment 1
[0071] Preparation of Compound 1
[0072]
[0073] step 1)
[0074]
[0075] Under nitrogen atmosphere, add reactant a-1 (5mmol) to 60mL anhydrous tetrahydrofuran (THF), add n-BuLi (5mmol) dropwise at -78°C, and keep it at -78°C Reaction for 2h; Compound A (5mmol) was dissolved in anhydrous THF, then added dropwise to the reaction solution, continued low temperature reaction for 1h, then warmed up to room temperature for overnight reaction. Treat that reaction finishes, add a small amount of water to quench, add dichloromethane / water (DCM / H 2 O) for extraction, the organic phase was collected and washed with anhydrous Na 2 SO 4 Dry, collect the filtrate by suction filtration, and spin off the solvent to obtain the crude product.
[0076] The above crude product was added to 30 mL of acetic acid under nitrogen, stirred and heated, and refluxed at 120° C. for 2 h, then 3 mL of hydrochloric acid was added, and heated at this temperature for 12 h. After the reaction was...
Embodiment 2
[0097] Preparation of Compound 2
[0098]
[0099]
[0100] The difference between the preparation method of this compound 2 and Example 1 is that the reactant 1 in the step (5) of Example 1 is replaced with an equimolar amount of reactant 2, and other raw materials, reaction steps and reaction conditions are the same as those in Example 1. 1 was the same, and compound 2 was finally obtained (yield 78%).
[0101] MALDI-TOF: m / z: Calculated: C 72 h 44 N 4 o 2 S: 1028.32, measured value: 1028.51.
[0102] Compound elemental analysis results: Calculated value: C 72 h 44 N 4 o 2 S (%): C 84.02, H 4.31, N 5.44; test value: C 84.01, H 4.30, N 5.46.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com