Industrial synthesis method of propofovir disoproxil fumarate
A technology of propofol fumarate and tenofovir, which is applied in the field of drug synthesis, can solve the problems of long reaction cycle and no significant improvement in the purity of intermediates, etc., to reduce the treatment of three wastes, use less solvent, and save production cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The preparation of embodiment 1 formula III compound
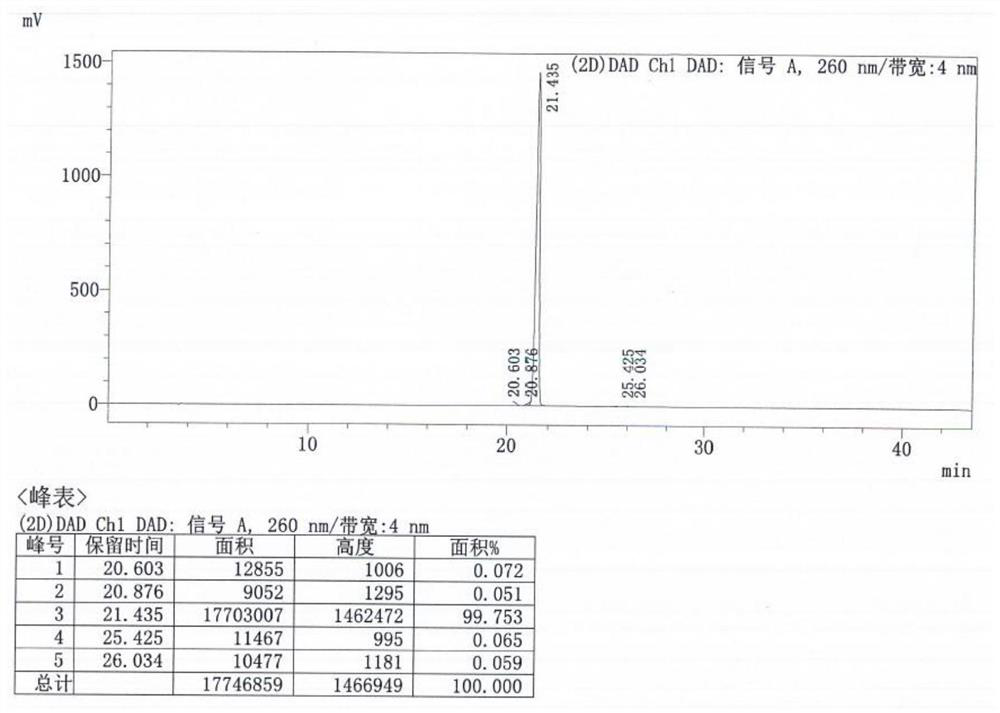
[0044] 1.0Kg formula PMPA, 2.2L of DMF, 704.3gTEA, 425.2gDMAP and 1619.7g Ph 3 o 3 Add P into a 10L three-necked flask, raise the temperature to 90-100°C, and react for 15-18 hours. After the reaction is complete, cool down to room temperature, add 5L of purified water, and wash the aqueous phase twice with ethyl acetate (3L×2). Adjust the pH of the water phase between 30°C to 2-3 with concentrated hydrochloric acid, cool down to 0-10°C, and crystallize for 2-3 hours. Centrifuge, wash the filter cake twice with dilute hydrochloric acid with pH=1.5, stir wash the wet filter cake with 7L ethyl acetate for 1-2 hours, centrifuge, and dry the obtained solid product in vacuum at 60-65°C for 24-26 hours to obtain 948.7g white solid That is formula III, the molar yield is 75.0%, and the HPLC purity is 99.75%.
Embodiment 2
[0045] The preparation of embodiment 2 formula III compound
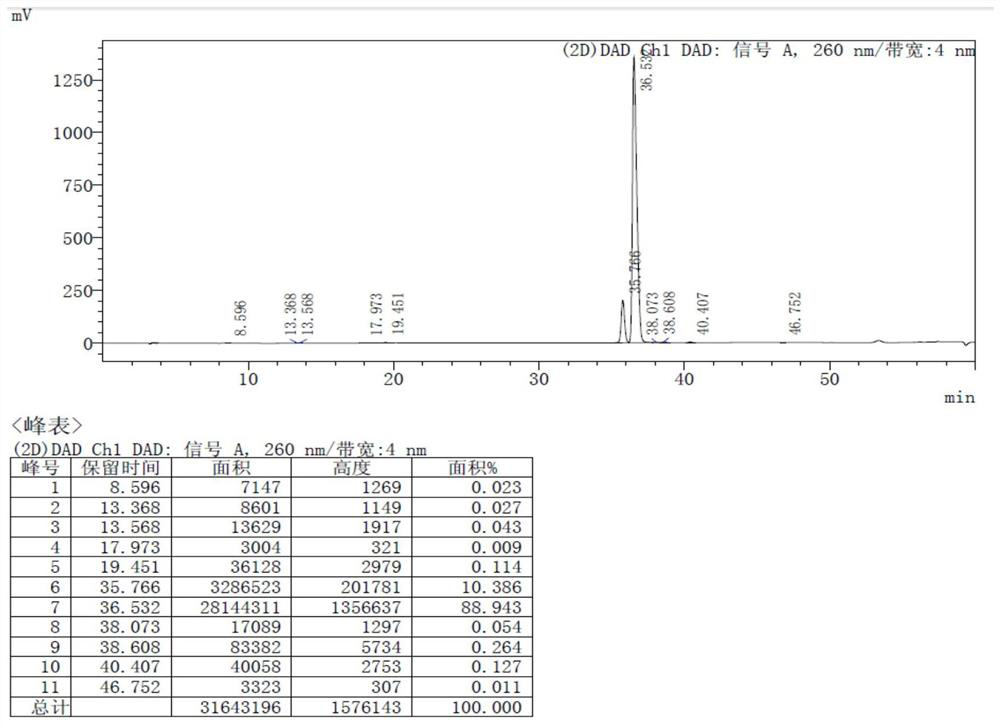
[0046] The DMSO of 1.0Kg formula PMPA, 2.2L, 704.3gTEA, 425.2gDMAP and 1619.7g Ph 3 o 3Add P into a 10L three-necked flask, raise the temperature to 90-100°C, and react for 15-18 hours. After the reaction is complete, cool down to room temperature, add 5L of purified water, and wash the aqueous phase twice with ethyl acetate (3L×2). Adjust the pH of the water phase between 30°C to 2-3 with concentrated hydrochloric acid, cool down to 0-10°C, and crystallize for 2-3 hours. Centrifuge, wash the filter cake twice with dilute hydrochloric acid with pH = 1.5, stir wash the wet filter cake with 7L ethyl acetate for 1-2 hours, centrifuge, and dry the solid product under vacuum at 60-65°C for 24-26 hours to obtain 910.8g white solid That is formula III, the molar yield is 72.0%, and the HPLC purity is 99.54%.
Embodiment 3
[0047] The preparation of embodiment 3 formula IV compound
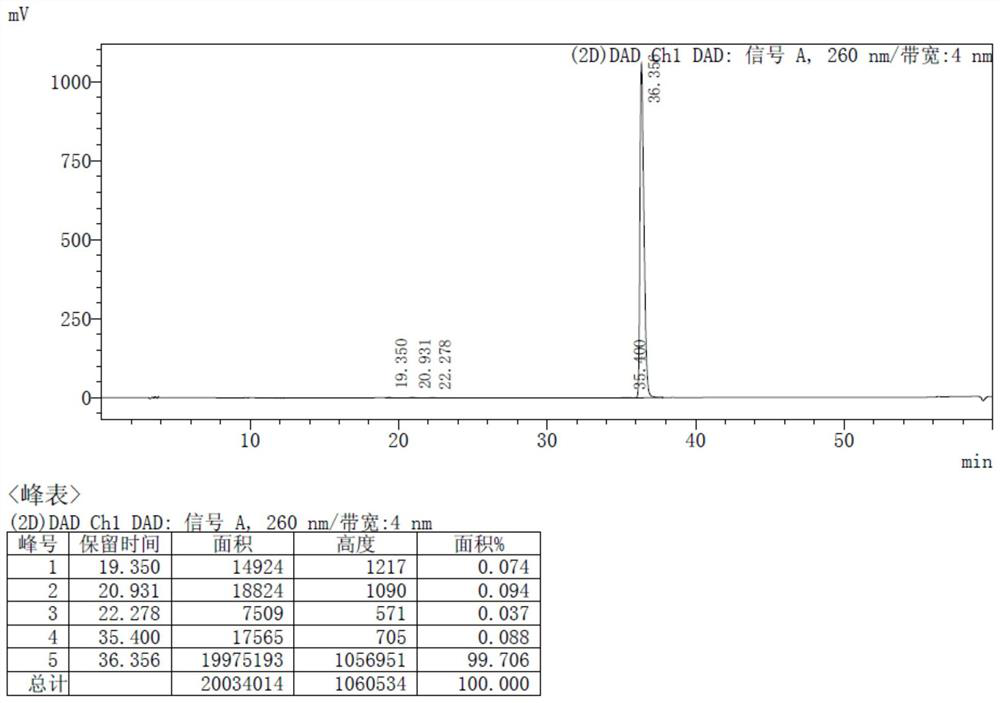
[0048] Add 800.0g of formula III and 6.4L of toluene into a 10L reaction flask, raise the temperature to an internal temperature of 75-80°C, and drop in 471.1g of SOCl 2 , Reaction 15~18h. The reaction solution was cooled to 50-60°C and concentrated until no distillate flowed out, added 6.4L of toluene, cooled to -25--20°C, added 922.0g of L-alanine isopropyl hydrochloride at one time, and dissolved 891.3g of TEA in 3.2 L of dichloromethane, slowly drop into the above reaction solution, temperature control -25 ~ -20 ℃, keep warm for 30 minutes after the drop is completed, warm up to room temperature, and react for 2 ~ 3 hours.
[0049] Post-processing process:
[0050] Add 4.8L of dichloromethane to the reaction solution and stir, the organic phase is washed twice with 10% sodium dihydrogen phosphate, washed once with saturated sodium chloride solution, dried over anhydrous sodium sulfate, filtered, and the filtrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com