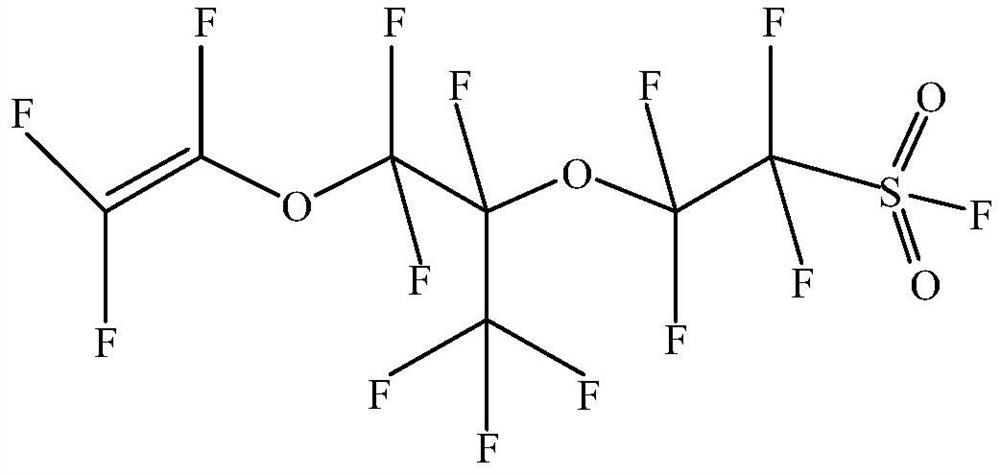
Preparation method of perfluoro-3, 6-dioxa-4-methyl-7-octenylsulfonyl fluoride
A technology of octenesulfonyl and dioxa, applied in the field of preparation of perfluoro 3,6-dioxa-4-methyl-7-octenesulfonyl fluoride, can solve the problem of many reaction steps and many by-products , long reaction time and other problems, to achieve the effect of short reaction route, less by-products, high yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
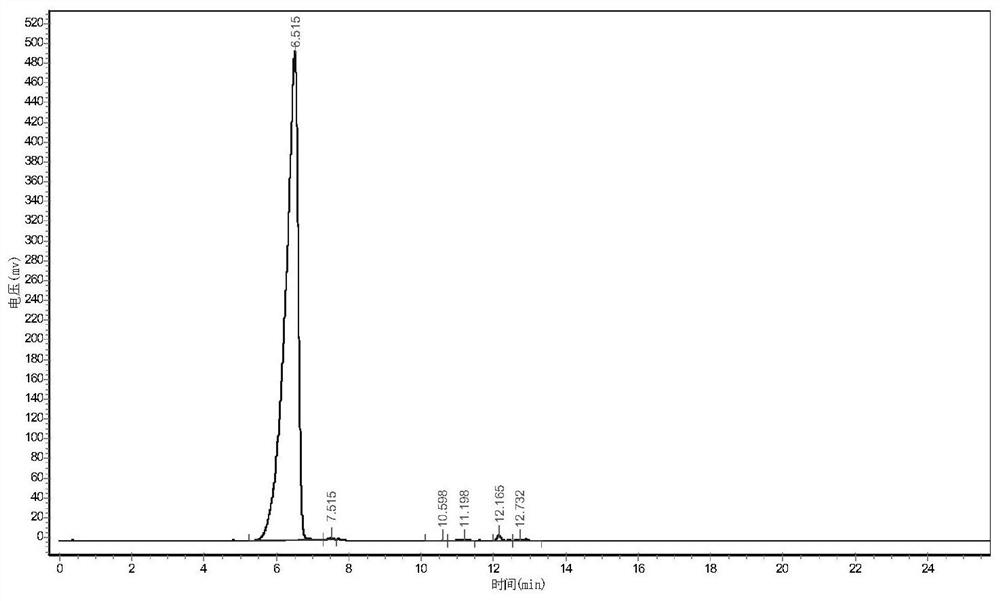
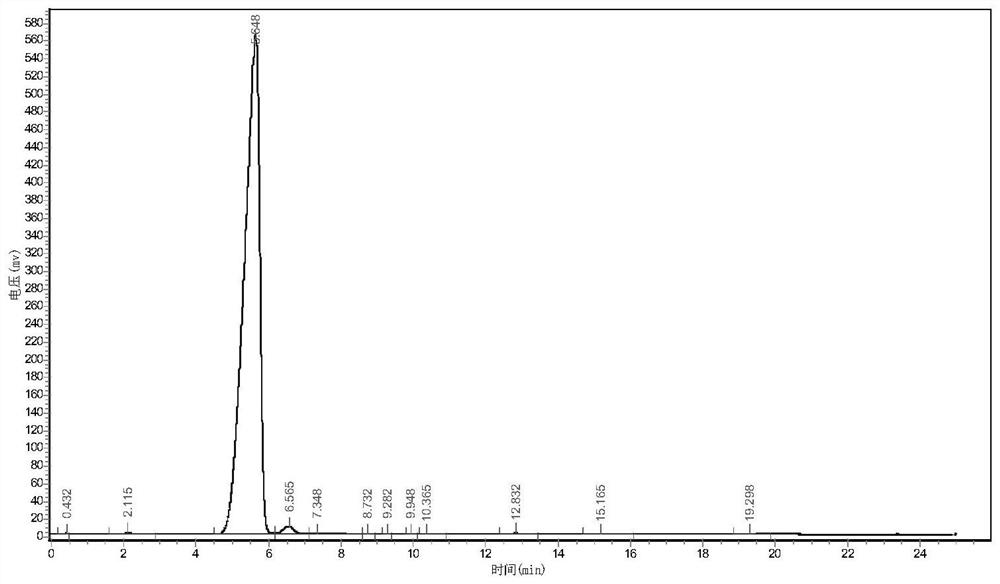
Image
Examples
Embodiment 1
[0032] (1) synthesis
[0033] In a 1L reactor, add ethylene glycol dimethyl ether 200ml, sodium fluoride 3.6g, tetrafluoroethane-β-sultone 180g (1mol), then import 300g (1.8mol) according to the flow rate of 0.25kg / h ) hexafluoropropylene oxide for addition reaction, the reaction temperature is controlled at 10°C, and the reaction pressure is controlled at 0.2MPa. After the introduction of hexafluoropropylene oxide is completed, the reaction is continued for 1h. After the reaction is completed, the reaction solution is rectified to obtain 478g ( 0.93mol) intermediate, the yield is 92%, and the purity is 99.3%;
[0034] (2) PSVE synthesis
[0035]In the moving bed reactor (volume 2L, material 316L, produced by Suzhou Newmt Technology Co., Ltd.), the mixture of 450g (0.87mol) of the intermediate obtained in step (1) and 400g of sodium carbonate (3.77mol) was passed through the moving The salt-forming zone and decarboxylation zone of the bed reactor (the salt-forming temperatur...
Embodiment 2
[0037] (1) Synthesis of intermediates
[0038] In a 1L reactor, add 50ml of diethylene glycol dimethyl ether, 10g of lithium fluoride, 200g (1.2mol) of tetrafluoroethane-β-sultone, and then import 250g (1.5mol) at a flow rate of 0.2kg / h. mol) of hexafluoropropylene oxide for addition reaction, the reaction temperature is controlled at 15°C, and the reaction pressure is controlled at 0.3MPa. After the introduction of hexafluoropropylene oxide is completed, the reaction is continued for 1h. After the reaction is completed, the reaction liquid is rectified to obtain 522g (1.01mol) intermediate, the yield is 91%, and the purity is 99.2%;
[0039] (2) PSVE synthesis
[0040] In the moving bed reactor (volume 2L, material 316L, produced by Suzhou Newmt Technology Co., Ltd.), the mixture of 500g (0.97mol) of the intermediate obtained in step (1) and 500g (4.71mol) of sodium carbonate is passed through the moving The salt-forming zone and decarboxylation zone of the bed reactor (the...
Embodiment 3
[0042] (1) Synthesis of intermediates
[0043] In a 1L reactor, add 300ml of dimethylformamide, 15.4g of calcium fluoride, 220g (1.22mol) of tetrafluoroethane-β-sultone, and then import 415g (2.5mol) at a flow rate of 0.1kg / h ) hexafluoropropylene oxide for addition reaction, the reaction temperature is controlled at 5°C, and the reaction pressure is controlled at 0.1MPa. After the introduction of hexafluoropropylene oxide is completed, the reaction is continued for 1h. After the reaction is completed, the reaction liquid is rectified to obtain 587g ( 1.15mol) intermediate, the yield is 93%, and the purity is 99.1%.
[0044] (2) PSVE synthesis
[0045] In a moving bed reactor (volume 2L, material 316L, produced by Kunshan Fuxi Engineering Technology Co., Ltd.), a mixture of 540g (1.05mol) of the intermediate obtained in step (1) and 550g (3.98mol) of potassium carbonate was passed through the moving The salt-forming zone and decarboxylation zone of the bed reactor (the salt-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com