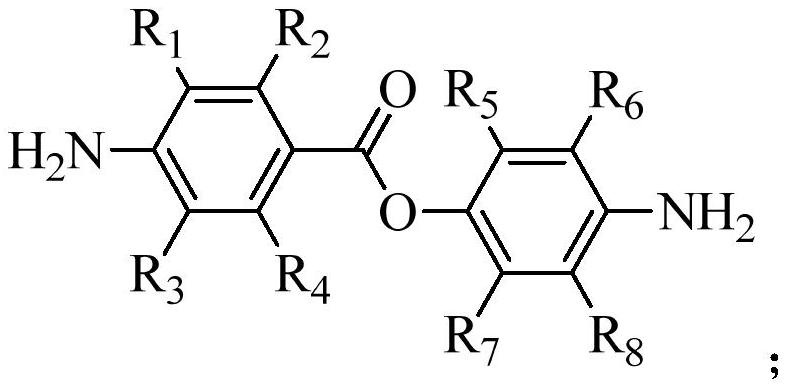
Trifluoromethyl substituted aromatic diamine compound containing aromatic ester structure and preparation method thereof
A technology of trifluoromethyl and aromatic diamine, applied in the field of trifluoromethyl substituted aromatic diamine compounds and their preparation, can solve the problems of reducing the heat resistance of materials, affecting the application range, etc., and achieves high heat resistance, excellent Effects of thermal stability and mechanical properties, good dissolution and film formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
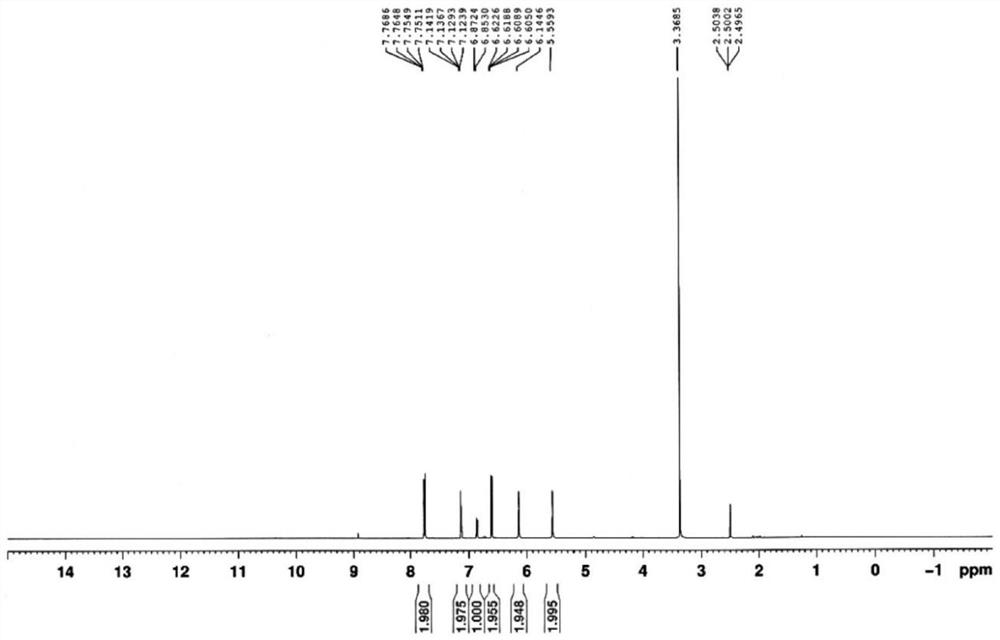
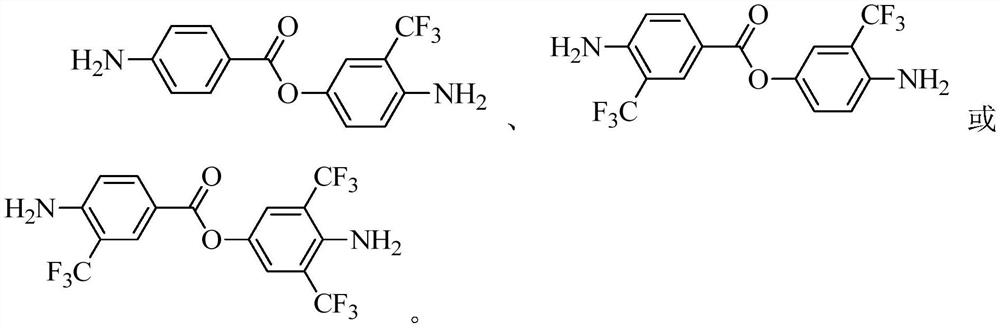
[0060]Example 1 Synthesis of 4,4'-diamino-3'-trifluoromethyl phenyl benzoate
[0061](1) Dissolve phenol (37.6g) in acetonitrile (1000ml), add sodium trifluoromethanesulfinate (CF3SO2Na) (31.2g), dichlorodicyanobenzoquinone (22.7g), 800mW / cm-2Irradiated with visible light, reacted at 25°C for 24h to obtain 3-trifluoromethylphenol (8.42g) with a yield of 13%.
[0062](2) Dissolve 3-trifluoromethylphenol (4.2g) in tetrahydrofuran, add Cu(NO3)2·3H2O (20.42g), react at 25°C for 6h to obtain 3-trifluoromethyl-4-nitrophenol (3.65g) with a yield of 68%.
[0063](3) Add 4-nitrobenzoic acid (4.00g) and 3-trifluoromethyl-4-nitrophenol (4.96g) into 90ml dichloromethane, add dicyclohexylcarbodiimide (DCC) ( 4.94g) and 4-dimethylaminopyridine (DMAP) (0.03g), reacted at 30℃ for 6h, filtered, concentrated and recrystallized to obtain 4,4'-dinitro-3'-trifluoromethyl phenyl benzoate (8.53g), the yield is 85%.
[0064](4) Add 4,4'-dinitro-3'-trifluoromethyl phenyl benzoate (10.00g) into 100ml tetrahydrofuran, ad...
Embodiment 2
[0067]Example 2 Synthesis of 4,4'-diamino-3,3'-bis(trifluoromethyl)phenyl benzoate
[0068](1) Combine benzoic acid (18.3g), trifluoroacetic acid (600ml), sodium thiosulfate (7.14g), titanium dioxide (TiO2) (4.8g) was added to the flask under 365nm ultraviolet light irradiation, reacted at room temperature for 24h, column chromatography separated to obtain 3-trifluoromethyl-benzoic acid (5.43g), the yield was 18%.
[0069](2) Dissolve 3-trifluoromethyl-benzoic acid (5.2g) in tetrahydrofuran, add Cu(NO3)2·3H2O (21.55g), react at 25°C for 6h to obtain 3-trifluoromethyl-4-nitrobenzoic acid (3.86g) with a yield of 60%.
[0070](3) Dissolve phenol (37.6g) in acetonitrile (1000ml), add sodium trifluoromethanesulfinate (CF3SO2Na) (31.2g), dichlorodicyanobenzoquinone (22.7g), 800mW / cm-2Irradiated with visible light, reacted at 25°C for 24h to obtain 3-trifluoromethylphenol (7.78g) with a yield of 12%.
[0071](4) Dissolve 3-trifluoromethylphenol (4.2g) in tetrahydrofuran, add Cu(NO3)2·3H2O (20.42g), re...
Embodiment 3
[0076]Example 3 Synthesis of 4,4'-diamino-2,3',6'-tris(trifluoromethyl)phenyl benzoate
[0077](1) Combine benzoic acid (18.3g), trifluoroacetic acid (600ml), sodium thiosulfate (7.14g), titanium dioxide (TiO2) (4.8g) was added to the flask under 313nm ultraviolet light irradiation, reacted at room temperature for 24h, column chromatography separated to obtain 3-trifluoromethyl-benzoic acid (4.56g), the yield was 16%.
[0078](2) Dissolve 3-trifluoromethyl-benzoic acid (5.2g) in tetrahydrofuran, add Cu(NO3)2·3H2O, react at 25°C for 6h to obtain 3-trifluoromethyl-4-nitrobenzoic acid (3.86g) with a yield of 60%.
[0079](3) Dissolve phenol (37.6g) in acetonitrile (1000ml), add sodium trifluoromethanesulfinate (CF3SO2Na) (62.4g), dichlorodicyanobenzoquinone (22.7g), 800mW / cm-2Visible light irradiation, reaction at 25°C for 24h to obtain 3,5-bis(trifluoromethyl)phenol (7.78g), with a yield of 12%.
[0080](4) Dissolve 3,5-bis(trifluoromethyl)phenol (4.2g) in tetrahydrofuran, add Cu(NO3)2·3H2O (20.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com