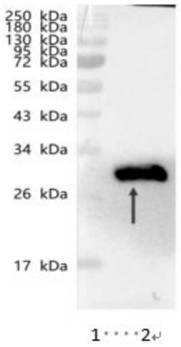
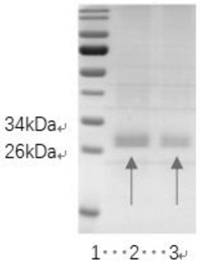
Truncated body based on novel coronavirus RBD-SD1 protein and application of truncated body
A coronavirus and protein technology, applied in the field of biomedicine, can solve problems such as production scale limitations, and achieve the effect of good safety and good immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] The acquisition of embodiment 1 recombinant plasmid
[0056] The recombinant vector is obtained by inserting the DNA molecule shown in SEQ ID No.1 into the pPIC9K SnaBI and NotI sites, entrusting Shanghai Jierui Bioengineering Co., Ltd. to obtain a recombinant plasmid.
[0057] The recombinant plasmid pPIC9K-T was sequenced and the result was correct.
Embodiment 2
[0058] Expression and purification of embodiment 2 recombinant protein T
[0059] Transformation and screening of yeast: After the expression vector pPIC9K-T was constructed, a large amount of plasmid DNA was extracted, and after linearization with BglII, 5 μg of the linearized plasmid was taken and transformed into competent Pichia pastoris GS115 by electroporation (P. pastoris GS115 strain was purchased from (from Invitrogen Company), the competent cells transformed by electroporation were spread on MD solid plates, and cultured upside down in a constant temperature incubator at 30°C for 2-3 days until the transformants grew out. Pick the transformant on the YPD+G418 (700μg / mL) medium, culture it at 30°C for 1-2 days, pick a larger white single colony, and inoculate it into a culture tube of 3mL BMGY medium, 30°C, 220rpm Cultivate on a shaking table for 48 hours, centrifuge the bacterial liquid cultured on a shaking table for 48 hours at 3000×g for 15 minutes, remove the sup...
Embodiment 3
[0062] Example 3 Determination of Antigen Immunological Activity
[0063] (1) Immunization method
[0064] grouping:
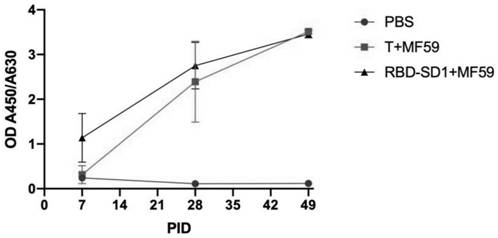
[0065] Animals 6-week-old Balb / C female mice were selected and divided into PBS group, RBD-SD1 protein+MF59 adjuvant group, T protein+MF59 adjuvant group, with 10 mice in each group.
[0066] Immunization method:
[0067] PBS group, RBD-SD1 protein + MF59 adjuvant group, T protein + MF59 adjuvant group, took leg muscle injection, immunized once every 3 weeks (immunized on day 0, day 21 and day 42 respectively), 10 μg protein / Rats (protein concentration 50 μg / mL), PBS group immunization volume of 200 μL / rat, a total of 3 times of immunization, the first immunization with MF59 adjuvant, mixed at 1:1.
[0068] Blood collection method and time:
[0069] Blood was collected from the tail vein, and the blood collection time was once before immunization, and once on the 7th day after each immunization.
[0070] (2) Determination of antibody titer in serum
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com