Carbonyl reductase mutant and application thereof
A reductase and mutant technology, applied in the field of bioengineering, can solve the problems of low atom economy, low activity and poor solubility of ethylsecodione, and achieve the effect of improved stereoselectivity, good activity and remarkable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 Construction of carbonyl reductase TbADH mutant library
[0027] According to the structure of TbADH, the non-conserved residues in its substrate binding pocket were selected for saturation mutation, and the mutation primers were designed using the degenerate codon NNK, and pET21a-TbADH was used as a template. The obtained monoclonal colonies were picked into 96-well deep-well plates for culture, and the expressed proteins were screened for high-throughput activity. The screening method was to use ethyl condensates as substrates and detect NADP(H )The change. The specific method of the reaction is: the substrate is dissolved in DMSO, the final concentration is 0.8mM, of which DMSO 60μL, NADPH 0.5mg / mL, crude enzyme solution 20μL, potassium phosphate buffer of pH 7.5 to 200μL, microplate reader detection For the decrease of NADPH at 340 nm, if the enzyme activity is relatively high, the consumption of NADPH will be faster, and the slope of the decline curve...
Embodiment 2
[0029] Example 2 Carbonyl reductase TbADH combinatorial mutant library construction
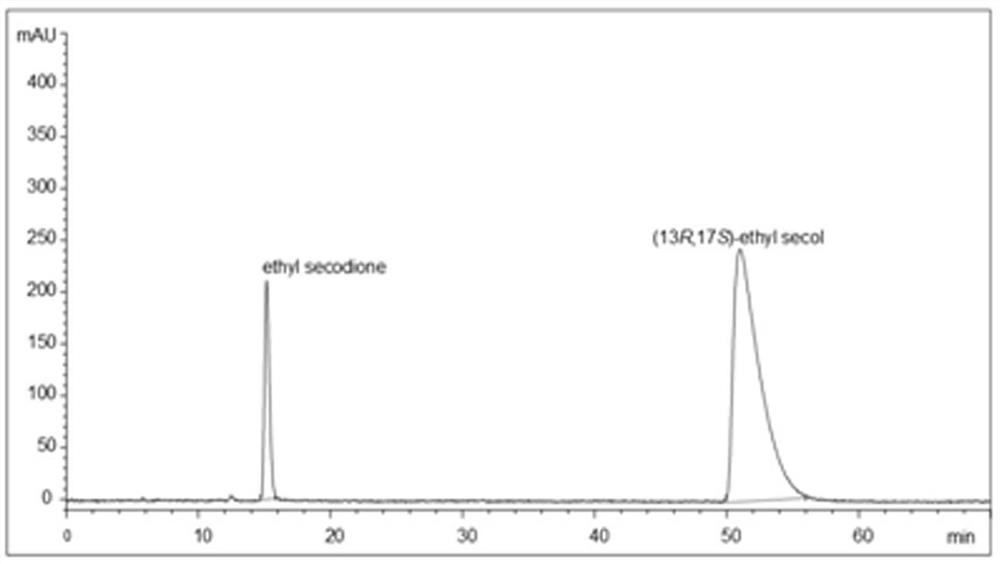
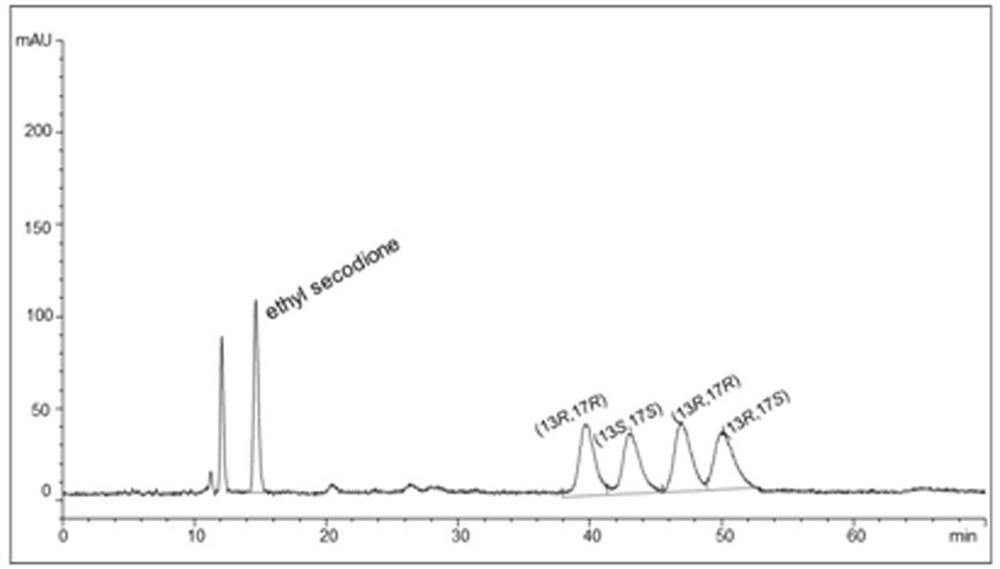
[0030] According to the results of saturation mutation, mutant 3 with improved activity and optimal stereoselectivity was selected as a template to construct combinatorial mutant libraries respectively (Table 1 shows the primer sequences used in the construction of mutant libraries). Experimental determination of the conversion of the ethyl condensate ethyl secodione (the specific operation was carried out as described in Example 4). The mutants obtained by the two-site combined mutation are mutants 4 and 5, and the protein sequences are SEQ ID NO: 5 and 6. Using mutant 4 as a template, the M285 mutation site is introduced, and the better mutant 6 protein obtained by screening The sequence is SEQ ID NO:7. The results are shown in Table 2.
[0031] Table 1 Primer sequences of mutant library
[0032]
[0033] Table 2 The conversion of ethyl secodione and the activity of isopropanol in the...
Embodiment 3
[0035] Example 3: Inducible expression of carbonyl reductase TbADH mutants
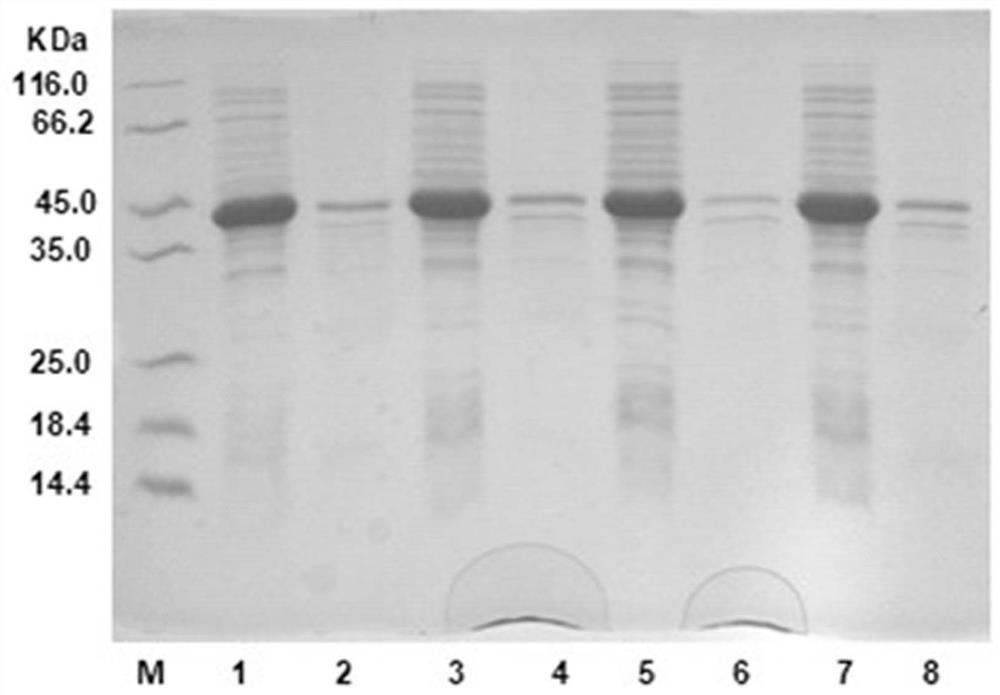
[0036] Prepare 50 mL of seed liquid, the medium is LB liquid medium (peptone 10 g / L, yeast powder 5 g / L, NaCl 10 g / L), pick a single colony of genetically engineered bacteria with an inoculation loop and insert it into the medium, 37 Cultivate overnight at 200 rpm. The overnight cultured seed solution was transferred to the fermentation medium (LB medium) at an inoculum of 1%, and cultivated to OD at 37°C and 200 rpm 600 0.6-1.0, add 0.1 mM IPTG, and place at 30°C, 200 rpm for 10-12 h. The bacterial cells were collected by centrifugation at 6000 rpm at 4°C and washed once with sodium phosphate buffer (100 mM, pH 7.0). SDS-PAGE electrophoresis pattern shows the induced expression of TbADH and mutants, such as figure 2 As shown, where, M represents Marker, 1 is TbADH wild-type protein expression supernatant, 2 is TbADH wild-type protein expression precipitate, 3 is representative mutant N114G protei...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com