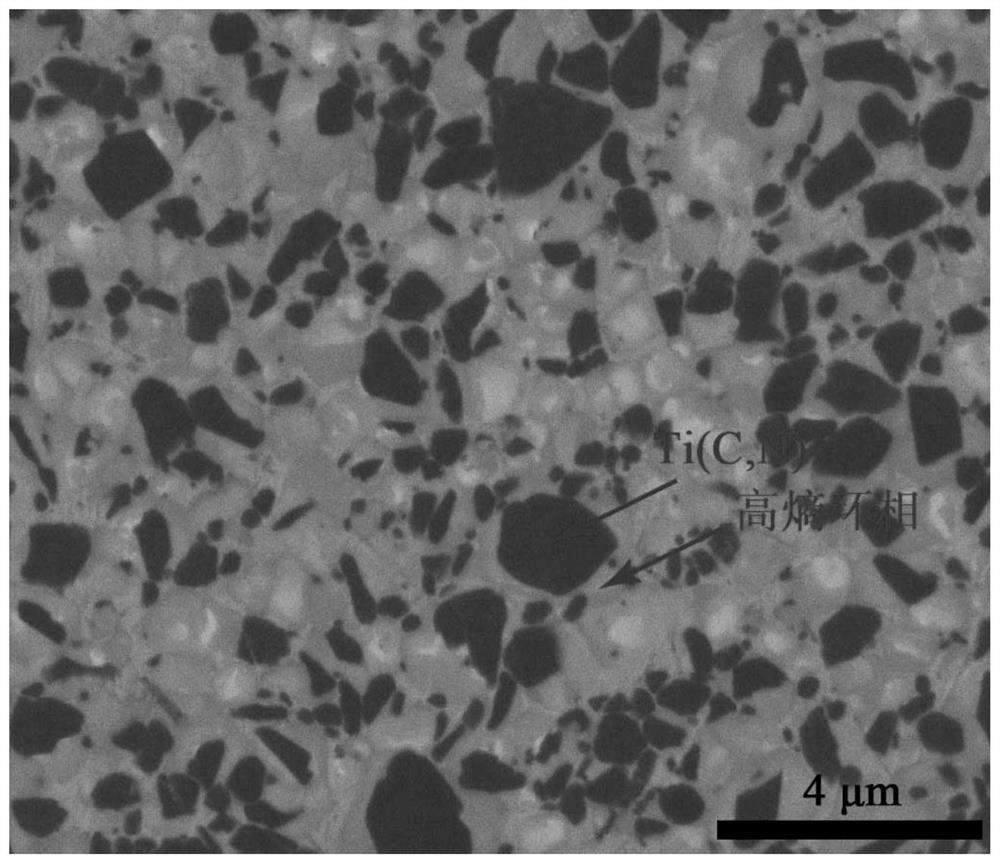
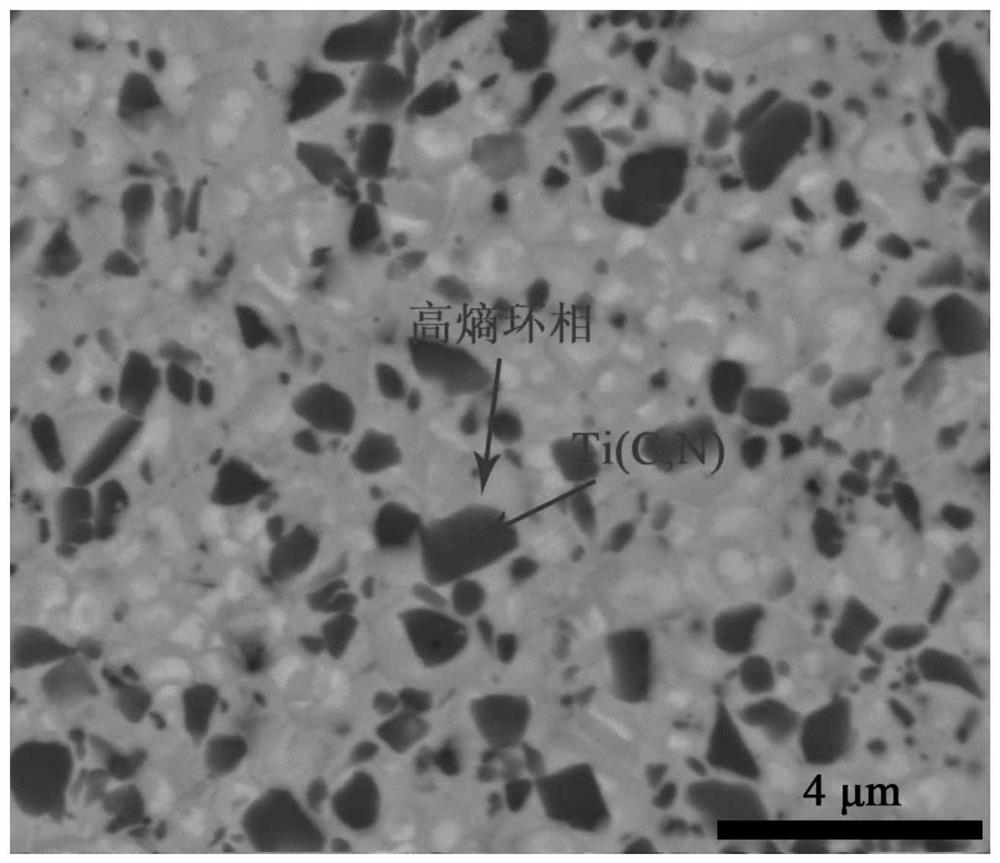
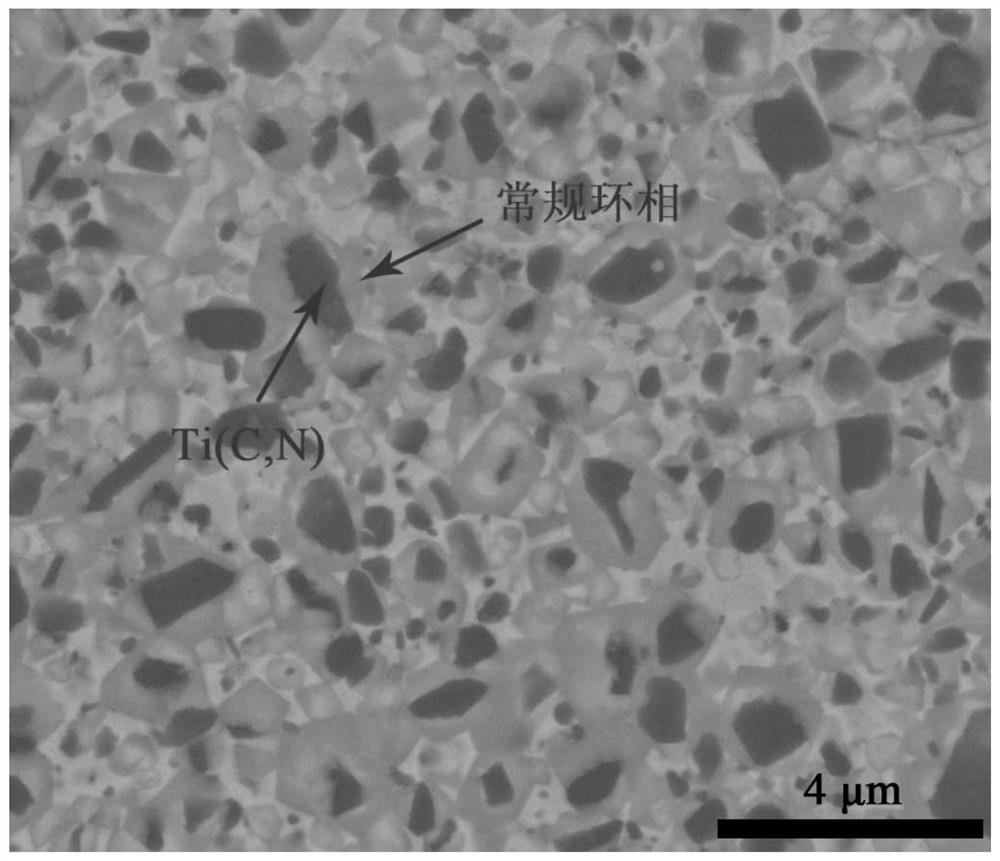
Ti (C, N)-based metal ceramic with high entropy ring phase structure and preparation method thereof
A technology of phase structure and base metal, applied in the direction of metal material coating process, coating, solid-state diffusion coating, etc., can solve the problems of reducing the surface hardness and wear resistance of the tool, reducing the surface processing accuracy, increasing the process and cost, etc. , to achieve excellent wear resistance and oxidation resistance, improve oxidation resistance and red hardness, good wear resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Composition: 49.9Ti(C,N)-11.4WC-6Mo 2 C-3.0VC-11.3TaC-18.0Ni-0.4C cermet.
[0045] The mass fraction of the cermet composition is 49.9 parts of Ti(C, N), 11.4 parts of WC, 6 parts of Mo 2 C, 3.0 parts of VC, 11.3 parts of TaC, 18.0 parts of binder phase Ni and 0.4 parts of carbon. The molar ratio of the four secondary carbides is: WC / Mo 2 C / VC / TaC=1.0 / 1.01 / 0.82 / 1.01.
[0046] First, the above secondary carbides and the corresponding oxides of the binder phase WO 3 / MoO 3 / V 2 o 5 / Ta 2 o 5 and NiO, and add pyrolytic carbon black required for complete carbothermal reduction, add cemented carbide balls, where the ball-to-material ratio is 20:1, and the ball milling time is 30h, and then the uniformly mixed powder is placed in an air atmosphere at 80°C Dry for 8 hours; put the mixed powder into a graphite calciner, and sinter at 1300°C for 2 hours in a high-purity argon atmosphere to obtain the desired nanocarbide-bonding phase composite powder.
[0047] The prep...
Embodiment 2
[0049] Composition: 40.7Ti(C,N)-15.5Mo 2 C-15.6ZrC-15.5NbC-12.5Co-0.2C cermet
[0050] The mass fraction of the cermet composition is 40.7 parts of Ti(C, N), 15.5 parts of Mo 2 C, 15.6 parts of ZrC, 15.5 parts of NbC, 12.5 parts of binder phase Co and 0.2 parts of carbon. Wherein the molar ratio of said 3 kinds of secondary carbides is: Mo 2 C / ZrC / NbC = 1.0 / 0.99 / 0.97.
[0051] First, the above-mentioned secondary carbides and the corresponding oxide MoO of the binder phase 3 / ZrO 2 / Nb 2 o 5 and CoO, and add pyrolytic carbon black required for complete carbothermal reduction, add cemented carbide balls, where the ball-to-material ratio is 25:1, and the ball milling time is 24h, and then the mixed powder is placed in an air atmosphere at 80°C Dry for 12 hours; put the mixed powder into a graphite calciner, and sinter at 1400°C for 3 hours in a high-purity argon atmosphere to obtain the desired nanocarbide-bonding phase composite powder.
[0052] The preparation steps of...
Embodiment 3
[0054] Element:
[0055] 32.1Ti(C,N)-21.5WC-10.7ZrC-10.1NbC-21.6TaC-3Ni-2Co-0.1C cermet.
[0056] The mass fraction of the cermet composition is 32.1 parts of Ti(C, N), 21.5 parts of WC, 10.7 parts of ZrC, 10.1 parts of VC, 21.6 parts of TaC, 3 parts of Ni, 2 parts of cobalt and 0.5 parts parts of carbon. Wherein the molar ratio of the four secondary carbides is: WC / ZrC / TaC / NbC=1.00 / 1.02 / 0.99 / 0.88.
[0057] First, the above secondary carbides and the corresponding oxides of the binder phase WO 3 / ZrO 2 / Ta 2 o 5 / Nb 2 o 5 / NiO / CoO, and adding pyrolytic carbon black required for complete carbothermal reduction, adding cemented carbide balls, wherein the ball-to-material ratio is 15:1, and the ball milling time is 48h, and then the mixed powder is placed in an air atmosphere, Dry at 80°C for 8 hours; put the mixed powder into a graphite calciner, and sinter at 1350°C for 5 hours in a high-purity argon atmosphere to obtain the desired nanocarbide-bonding phase composite p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com