Near-infrared fluorescent compound for specific detection of hydrazine and preparation method of near-infrared fluorescent compound
A fluorescent compound and detection method technology, applied in the field of organic small molecule fluorescent probes, can solve problems such as time-consuming operation and complexity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
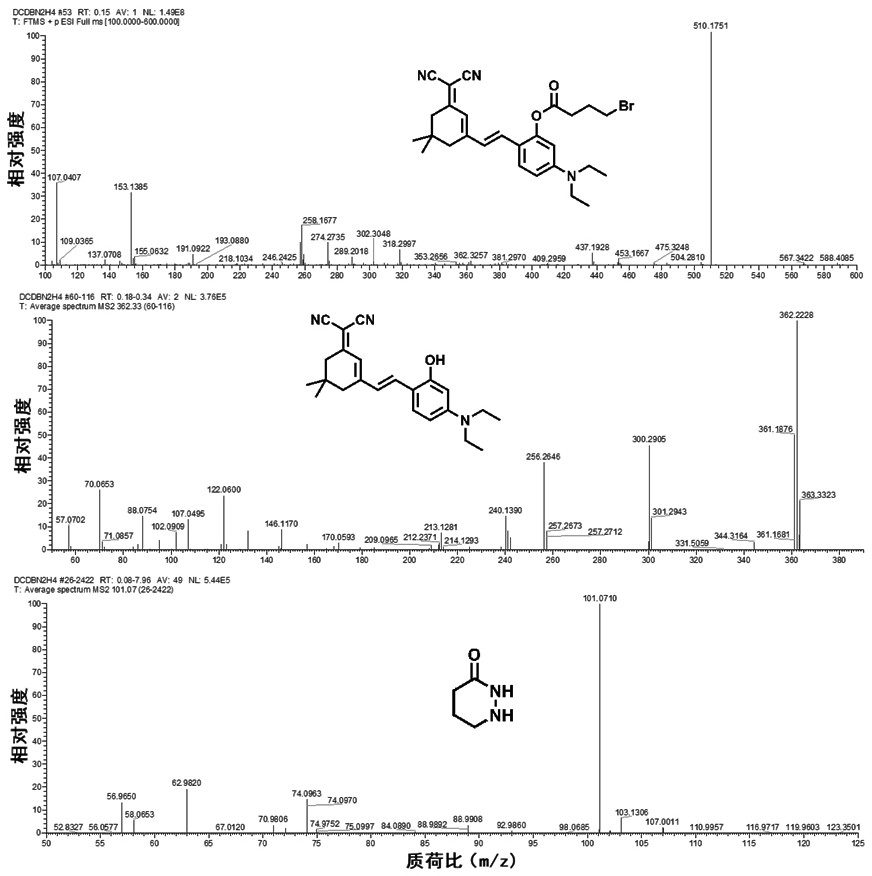
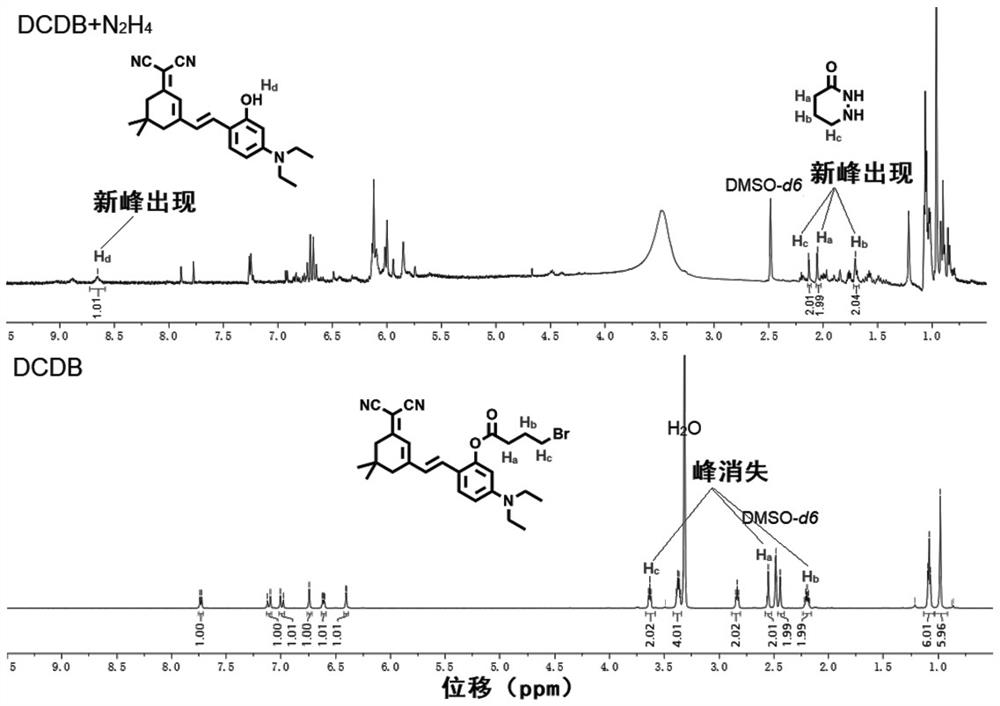
[0072] Preparation of Near Infrared Fluorescent Compounds for Hydrazine Detection
[0073] The near-infrared fluorescent compound of the present invention is (E)-2-(2-(3-(dicyanomethylene)-5,5-dimethylcyclohex-1-en-1-yl) vinyl )-5-(diethylamino)phenyl 4-bromobutanoic acid (DCDB), the specific preparation process is as follows:
[0074]
[0075] (1) Under nitrogen protection, DHDM (1.0g, 2.8mmol), triethylamine (1.0mL) and anhydrous dichloromethane (50mL) were mixed and added to a 100mL three-necked flask, cooled to 0 with an ice bath ℃.
[0076] (2) Under ice-bath conditions, then slowly add 4-bromobutyryl chloride (0.62mg, 3.3mmol) dropwise into the three-necked flask containing the mixture in step (1), and stir at room temperature for 10-12 hours. The progress of the reaction was monitored using thin layer chromatography (TLC) during the reaction.
[0077] (3) After the reaction was completed, the mixture was poured into 50 mL of ice water at 0° C., stirred and mixed, ...
Embodiment 2
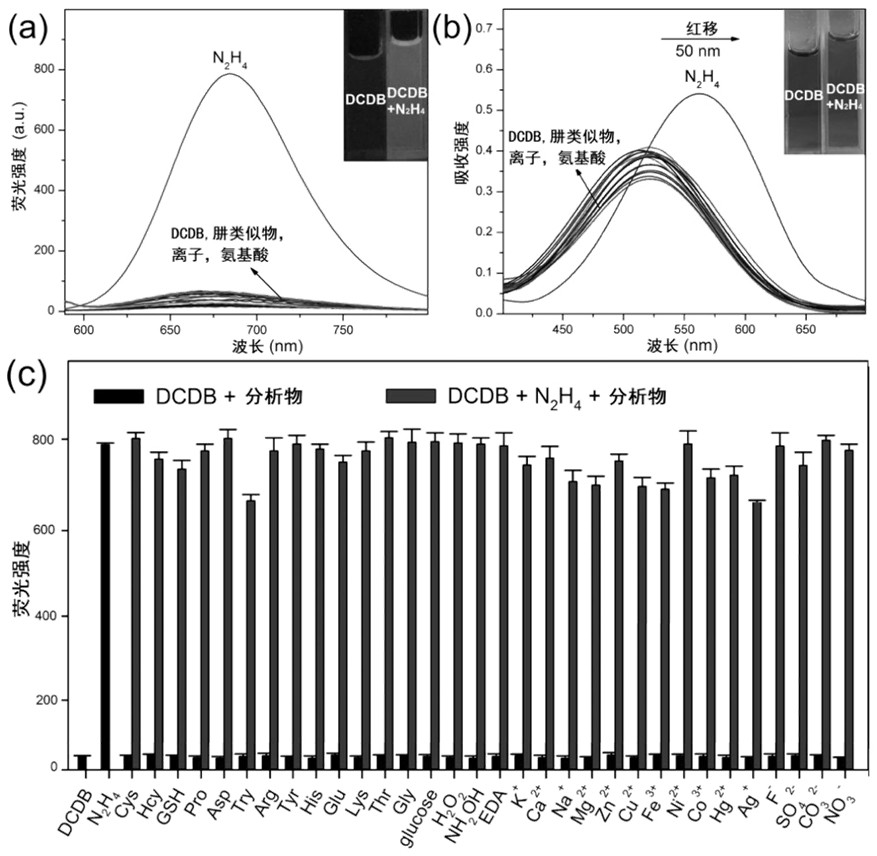
[0090] Selective detection of hydrazine in solution system
[0091] (1) The buffer solution for the near-infrared fluorescent compound prepared in Example 1 is prepared into a near-infrared fluorescent compound solution with a concentration of 10 μM, and the buffer solution is composed of phosphate buffered saline (PBS) with a volume ratio of 1:1 and Dimethyl sulfoxide (DMSO) is prepared, and the pH value of the buffer is 7.4;
[0092] (2) hydrazine in the solution is selectively detected with a 10 μM near-infrared fluorescent compound solution;
[0093] Take 81 parts of 3mL near-infrared fluorescent compound solution with a concentration of 10μM, and add 120μL of a concentration of 5×10 -5 mol / L of analytes to be measured, there are 27 kinds of analytes to be measured, and each analyte to be measured is composed of 3 parallel units to form a group, and a total of 81 reactants are obtained; 27 kinds of analytes to be measured are 200 μ M hydrazine ( N 2 h 4 ), 200 μM magne...
Embodiment 3
[0097] Detection Sensitivity of Near-infrared Fluorescent Compounds to Hydrazine in Solution System
[0098] (1) The buffer solution for the near-infrared fluorescent compound prepared in Example 1 is prepared into a near-infrared fluorescent compound solution with a concentration of 10 μM, and the buffer solution is composed of phosphate buffered saline (PBS) with a volume ratio of 1:1 and Dimethyl sulfoxide (DMSO) is prepared, and the pH value of the buffer is 7.4;
[0099] (2) Use 10 μM near-infrared fluorescent compound solution to test hydrazine at different concentrations (0 μM, 10 μM, 20 μM, 30 μM, 40 μM, 50 μM, 60 μM, 70 μM, 80 μM, 90 μM, 100 μM, 110 μM, 120 μM, 130 μM, 140 μM, 150 μM) Fluorescence detection;
[0100] (3) Add different concentrations (0 μM, 10 μM, 20 μM, 30 μM, 40 μM, 50 μM, 60 μM, 70 μM, 80 μM, 90 μM, 100 μM, 110 μM, 120 μM to 48 prepared 3 mL near-infrared fluorescent compound solutions with a concentration of 10 μM, respectively. , 130 μM, 140 μM,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com