A method for preparing coronene compounds from acyclic olefins
A non-cyclic, compound technology, applied in the direction of hydrocarbons, hydrocarbons, carbon compound catalysts, etc., can solve the problems of unstable intermediate products and inconvenient industrialization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1: the synthesis of RHO molecular sieve catalyst
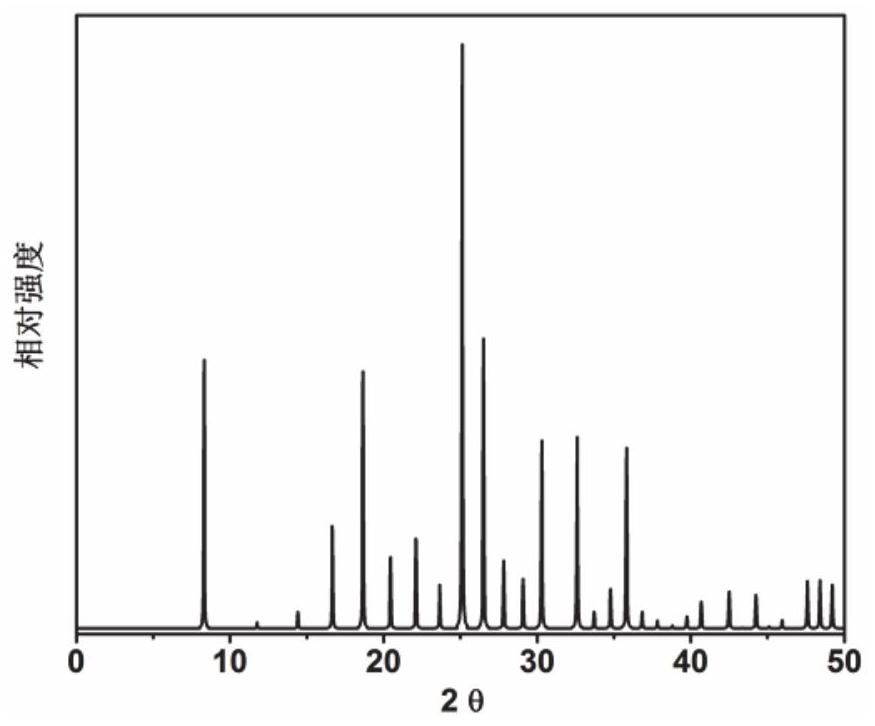
[0053] Initial gel molar composition ratio 2N-methylbutylamine (as organic structure directing agent): 3Na 2 O: 0.4Cs 2 O: Al 2 o 3 : 10SiO 2 : 110H 2 O Mix the measured sodium oxide, cesium oxide, pseudoboehmite, silica sol and deionized water in a beaker, stir well to form a gel, then put it into a stainless steel autoclave lined with polytetrafluoroethylene, and 100 ℃ constant temperature 50h. After crystallization, use a centrifuge at 3000 rpm for 3 minutes, wash the separated solid product with deionized water to neutrality, and dry it overnight in air at 120°C. The results of XRD analysis are as follows: figure 1 shown. from figure 1 It can be seen from the results that the synthesized product is the raw powder of RHO molecular sieve, which is calcined at 550°C for 5 hours to obtain RHO molecular sieve, which is designated as catalyst 1.
Embodiment 2
[0054] Embodiment 2: the synthesis of ITQ-29 molecular sieve catalyst
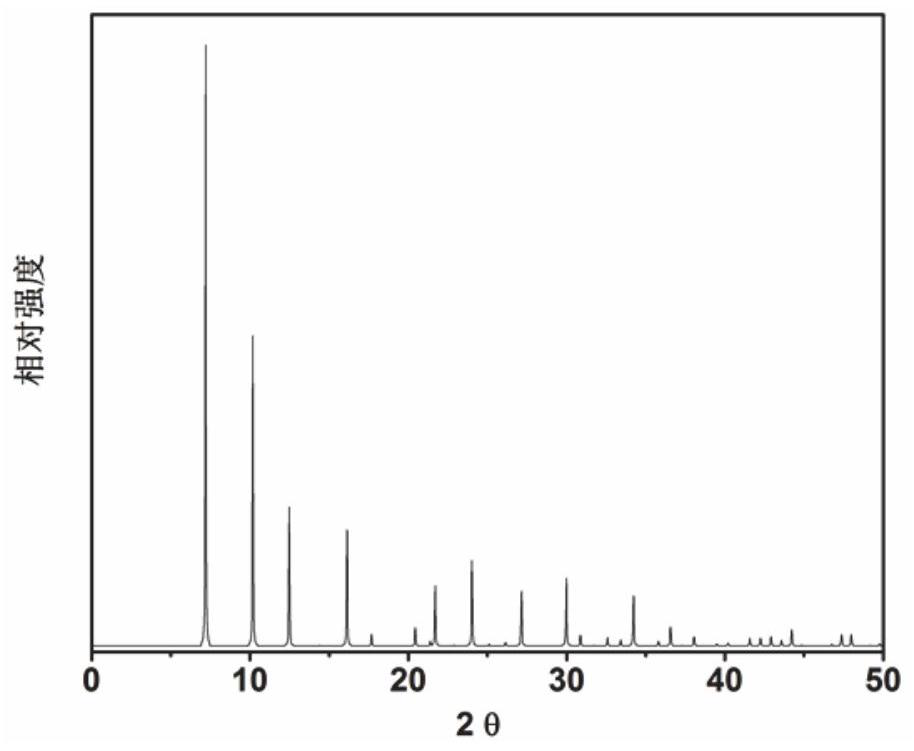
[0055] Based on the initial gel molar composition ratio of 1.85 2,2-dimethyl-2,3-dihydro-1H-phenyl[de]isoquinoline (as an organic structure-directing agent): 0.2SiO 2 : 0.8GeO 2 : 0.06Al 2 o 3 : 0.25 4-Methyl-2,3,6,7-tetrahydro-1H, 5H-pyridine[3,2,1-ij]quinoline: 0.25 Tetramethylamine: 0.5HF: 6H 2 O Mix measured amounts of silica sol, germanium dioxide, aluminum isopropoxide, organic structure-directing agent, hydrofluoric acid and deionized water in a beaker, stir well to form a gel, and then put it into a polytetrafluoroethylene-lined In a stainless steel autoclave, crystallize at a constant temperature of 135°C for 6 days. After crystallization, use a centrifuge at 3000 rpm for 3 minutes, wash the separated solid product with deionized water until it is neutral, and dry it overnight in air at 100°C. The results of XRD analysis are as follows: figure 2 shown. from figure 2 It can be seen from th...
Embodiment 3
[0056] Embodiment 3: the synthesis of UZM-9 molecular sieve catalyst
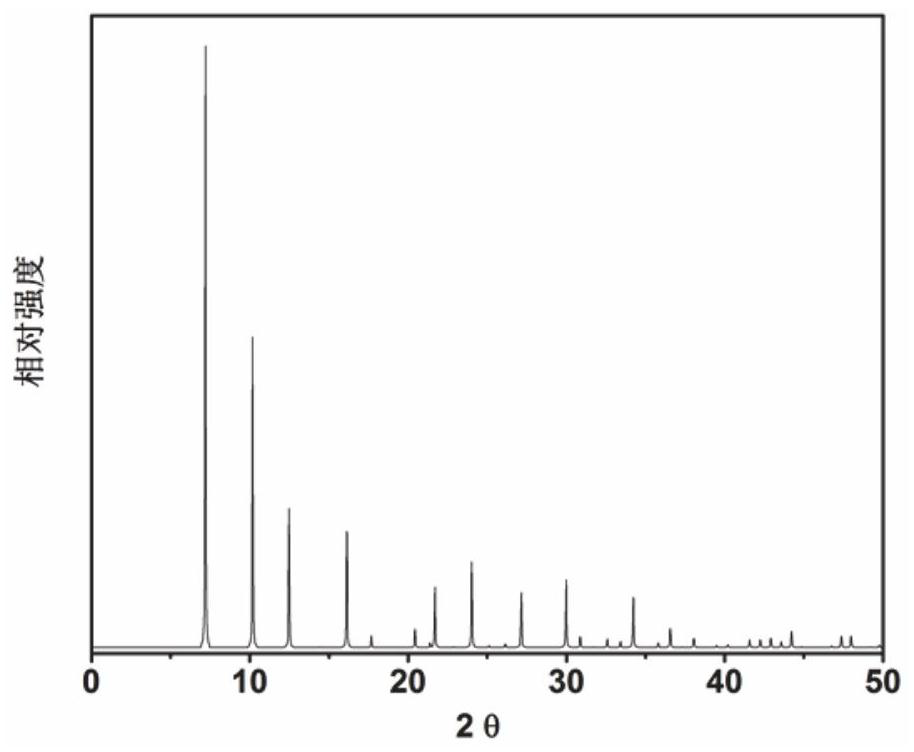
[0057] In the initial gel molar composition ratio 9 tetraethylammonium hydroxide: 2 tetramethylammonium hydroxide: 0Na 2 O: Al 2 o 3 : 16SiO 2 : 62H 2 O mix metered tetraethylammonium hydroxide, tetramethylammonium hydroxide (organic structure-directing agent), aluminum isopropoxide, silica sol and deionized water in a beaker, fully stir to form a gel, then pack into Crystallize at room temperature for 6 hours in a stainless steel autoclave lined with polytetrafluoroethylene. After crystallization, use a centrifuge at 3000 rpm for 3 minutes, wash the separated solid product with deionized water until it is neutral, and dry it overnight in air at 100°C. The results of XRD analysis are as follows: image 3 shown. from image 3 It can be seen from the results that the synthesized product is the original powder of UZM-9 molecular sieve, which is calcined at 600°C for 5 hours to obtain UZM-9 molecular sie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com