Paclitaxel and lapatinib compound nanocrystal and preparation method thereof
A paclitaxel lapatinib and nanocrystal technology, which can be applied in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. problem, to achieve the effect of good water dispersibility, improved stability, and easy targeted modification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Preparation of paclitaxel / lapatinib compound nanocrystals
[0038] Dissolve 1mg of paclitaxel, 2mg of lapatinib, 3mg of vitamin E polyethylene glycol 1000 succinate and 6mg of citric acid in an appropriate amount of absolute ethanol, place in a pear-shaped bottle for rotary evaporation, evaporate the ethanol, and form a thin film at the bottom of the bottle , add 5mL of sodium bicarbonate solution (4mg / mL), shake gently at room temperature for hydration, centrifuge the resulting suspension at 8000rpm for 15 minutes, discard the supernatant, collect the precipitate, and disperse with pure water to obtain Paclitaxel / lapatinib compound nanocrystal dispersion with light blue opalescence;
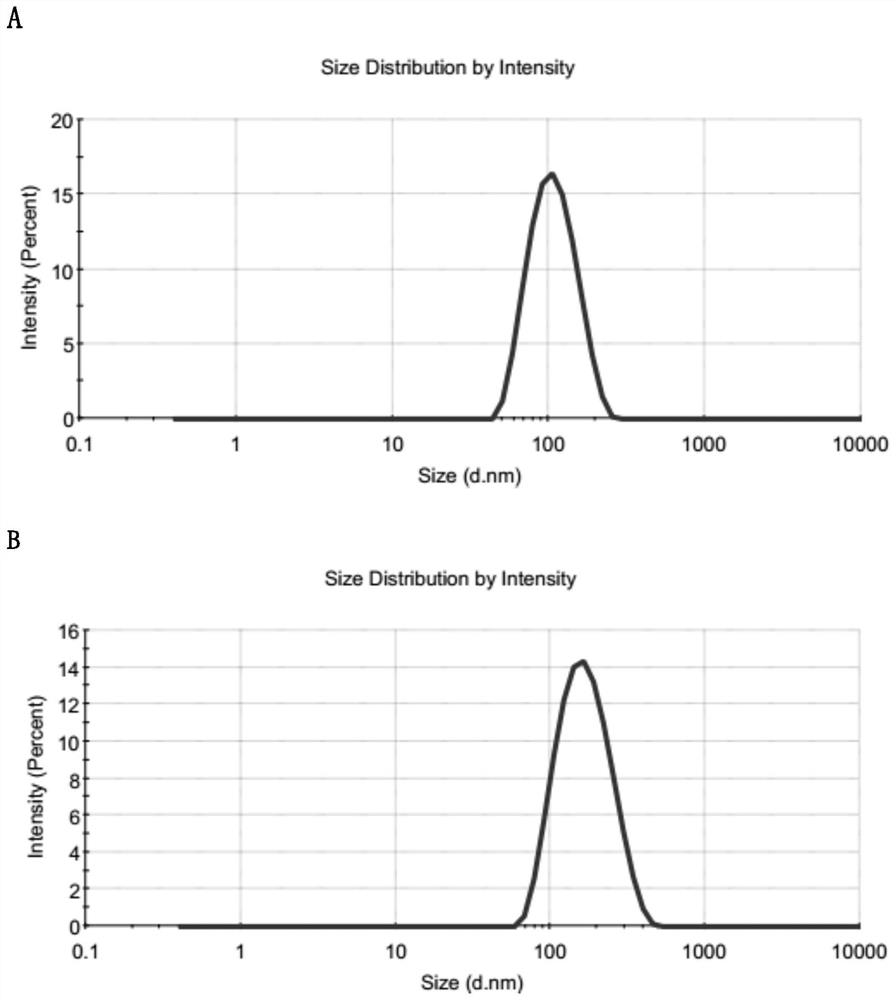
[0039] Analyze its particle size distribution with the dynamic light scattering method, the average particle size is 92nm, and the polydispersity coefficient (PDI 0.076) (such as figure 1shown); the so-called "polydispersity coefficient" is the particle polydispersity parameter...
Embodiment 2
[0046] Example 2 Preparation of phospholipid-coated paclitaxel / lapatinib compound nanocrystals
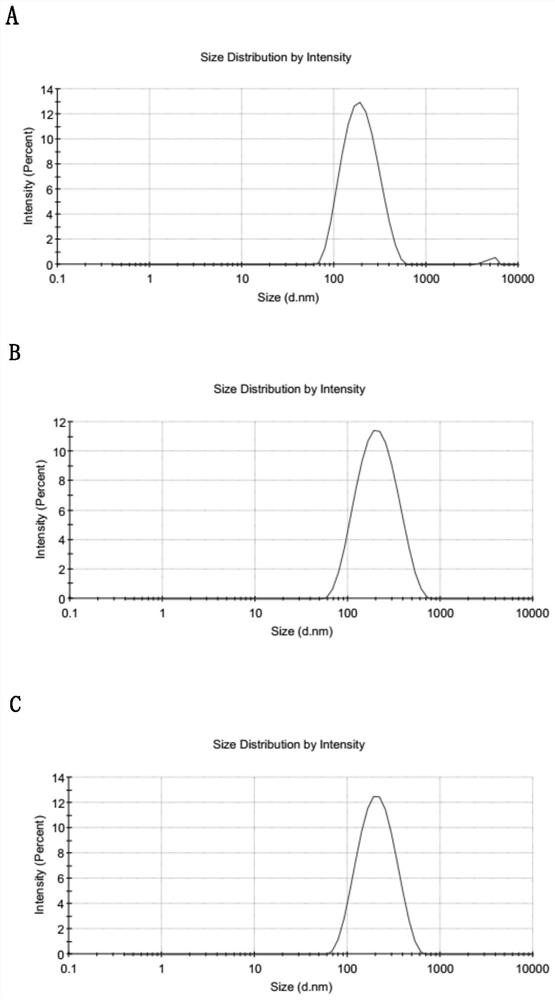
[0047] Weigh lecithin (1.15mg), cholesterol (0.58mg), octadecylamine (0.36mg), vitamin E macrogol 1000 succinate (0.58mg), phospholipid-polyethylene glycol-methoxy (0.70 mg) was dissolved in an appropriate amount of chloroform, placed in a pear-shaped bottle for rotary evaporation, and the chloroform was waved off to form a thin film at the bottom of the bottle. Add the paclitaxel / lapatinib compound nanocrystals described in Example 1, and gently shake Hydration, the resulting suspension was centrifuged at 8000rpm for 10 minutes to remove unreacted phospholipids to obtain phospholipid-coated paclitaxel / lapatinib compound nanocrystals (transmission electron microscope photos as shown in figure 1 Shown in D), average particle size 102nm, PDI 0.153 ( figure 2 B), stored at 25°C for 6 months, the particle size is basically unchanged, and the stability is good (such as image 3 shown...
Embodiment 3
[0052] Example 3 Preparation of folic acid-modified phospholipid-coated paclitaxel / lapatinib compound nanocrystals
[0053] The steps are the same as in Example 2, the difference is that the phospholipid material consists of lecithin (1.15mg), cholesterol (0.58mg), octadecylamine (0.36mg), vitamin E polyethylene glycol 1000 succinate (0.58mg) , folic acid-modified phospholipid-polyethylene glycol (0.70mg);
[0054] Cytotoxicity tests carried out on breast cancer drug-resistant cell lines (MCF-7 / ADR) showed that folic acid-modified phospholipid-coated paclitaxel / lapatinib compound nanocrystals had better inhibitory effects on tumor cell growth than those described in Example 2 The median lethal concentrations of phospholipid-coated paclitaxel / lapatinib compound nanocrystals, phospholipid-coated paclitaxel / lapatinib compound nanocrystals (without folic acid modification) and folic acid-modified phospholipid-coated paclitaxel / lapatinib compound nanocrystals (IC 50 ) are 1.7×10 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com