Preparation method of decellularized amnion intrauterine scaffold carrying estradiol microspheres
An estradiol-loaded, decellularized technology, applied in the field of biomedical equipment preparation, can solve the problems of high recurrence rate and poor treatment effect of intrauterine adhesions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
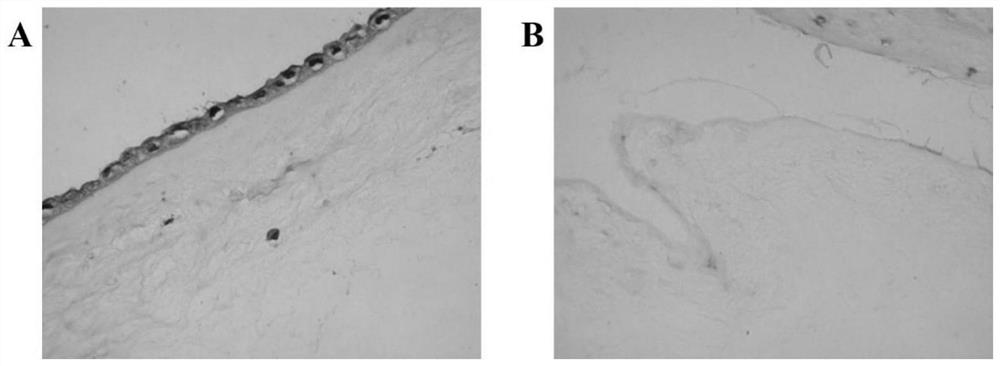
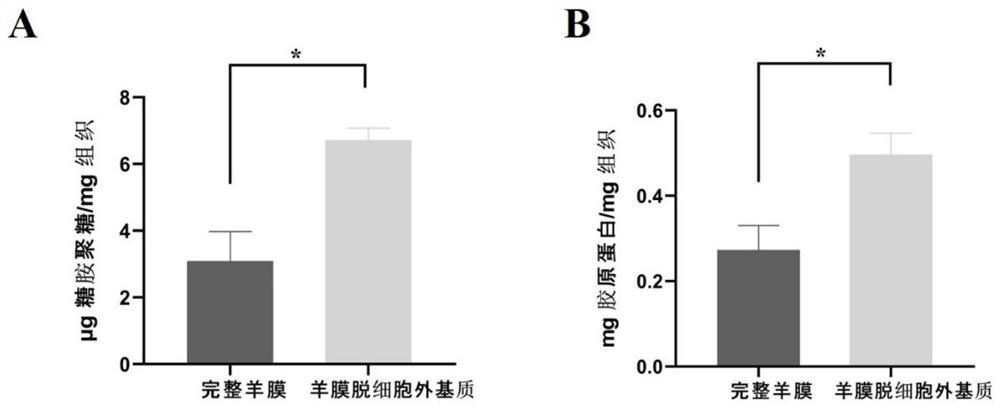
[0028] A. Preparation of acellular amnion matrix:
[0029] (1) Take amniotic membrane tissue: take the placenta negative for hepatitis B, hepatitis C, AIDS, and syphilis under sterile conditions, bluntly separate the amniotic membrane about 10cm×10cm, remove the chorionic membrane tissue, and use 50mg / ml penicillin, 50mg / ml streptomycin Stored after washing with phosphate buffered saline (pH 7.2-7.4).
[0030] (2) Preparation of acellular amniotic matrix: the amniotic membrane was washed repeatedly with normal saline, soaked in the volume fraction polyethylene glycol octylphenyl ether (TritonX-100) solution, placed in a constant temperature incubator at 37°C and shaken overnight, and taken out Then rinse with normal saline, add 25g / L trypsin and 0.2g / L ethylenediaminetetraacetic acid (EDTA), fully shake, and incubate at 37°C for 4h. After taking it out, rinse it repeatedly with normal saline, then place the amniotic membrane tissue in 8.8% sodium chloride solution, incubate a...
Embodiment 2
[0050] A. Preparation of acellular amnion matrix:
[0051] (1) Take amniotic membrane tissue: take the placenta negative for hepatitis B, hepatitis C, AIDS, and syphilis under sterile conditions, bluntly separate the amniotic membrane about 10cm×10cm, remove the chorionic membrane tissue, and use 50mg / ml penicillin, 50mg / ml streptomycin Stored after washing with phosphate buffered saline (pH 7.2-7.4).
[0052] (2) Preparation of acellular amnion matrix: the amnion was rinsed repeatedly with normal saline, soaked in 0.1% TritonX-100 solution by volume fraction, placed in a constant temperature incubator at 37°C and shaken overnight, rinsed with normal saline after taking it out, and added 25g / L trypsin and 0.2g / L EDTA, shake well, and incubate at 37°C for 4h. After taking it out, rinse it repeatedly with physiological saline, then place the amnion tissue in 8.8% sodium chloride solution, incubate at room temperature for 4 hours, take it out, wash it repeatedly with 0.01M phos...
Embodiment 3
[0075] A. Preparation of acellular amnion matrix:
[0076] (1) Take amniotic membrane tissue: take the placenta negative for hepatitis B, hepatitis C, AIDS, and syphilis under sterile conditions, bluntly separate the amniotic membrane about 10cm×10cm, remove the chorionic membrane tissue, and use 50mg / ml penicillin, 50mg / ml streptomycin Stored after washing with phosphate buffered saline (pH 7.2-7.4).
[0077] (2) Preparation of acellular amnion matrix: the amnion was rinsed repeatedly with normal saline, soaked in 0.1% TritonX-100 solution by volume fraction, placed in a constant temperature incubator at 37°C and shaken overnight, rinsed with normal saline after taking it out, and added 25g / L trypsin and 0.2g / L EDTA, shake well, and incubate at 37°C for 4h. After taking it out, rinse it repeatedly with physiological saline, then place the amnion tissue in 8.8% sodium chloride solution, incubate at room temperature for 4 hours, take it out, wash it repeatedly with 0.01M phos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com