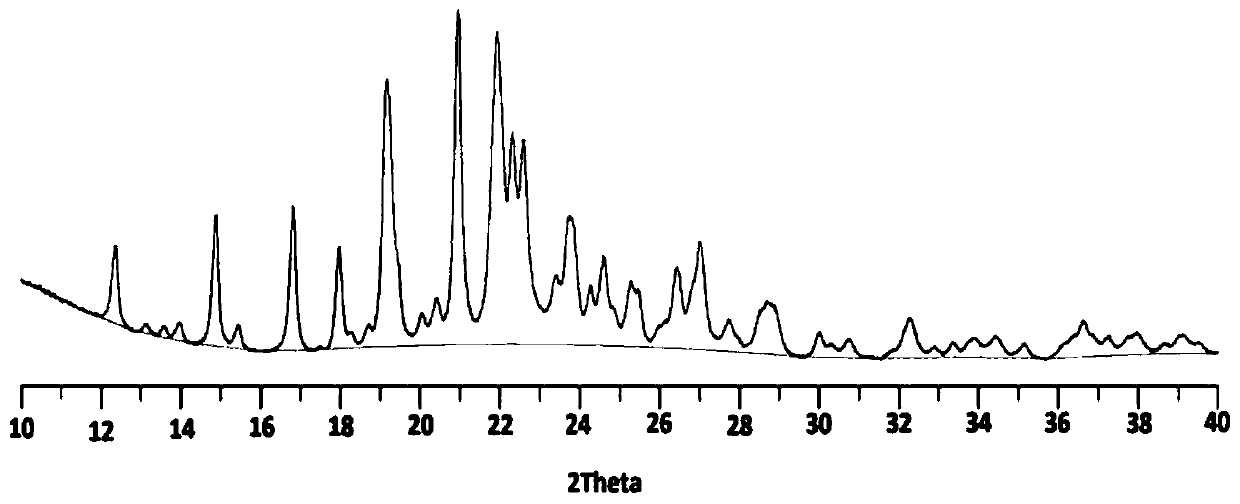
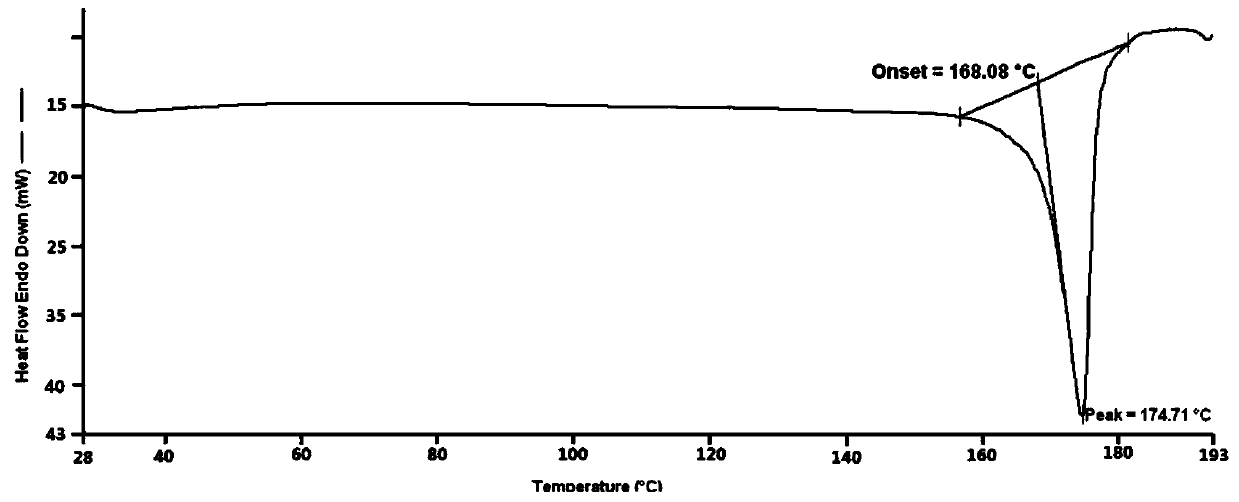
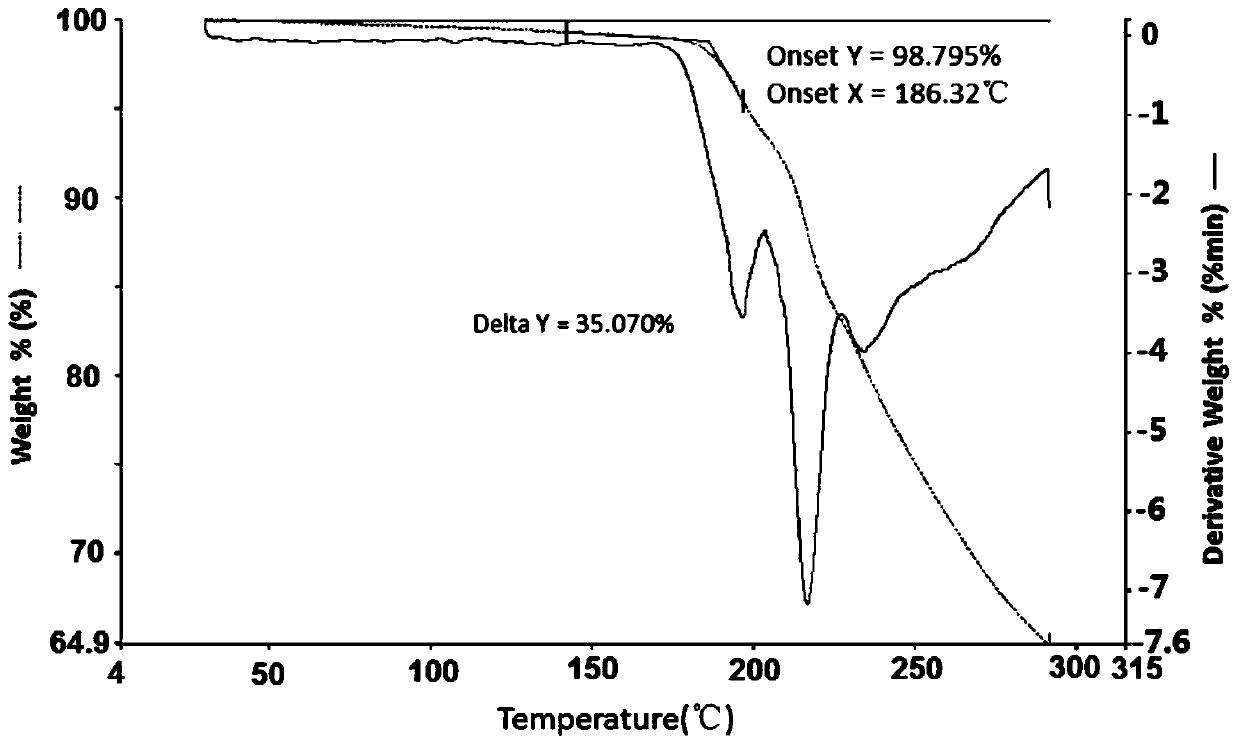
Famotidine-malic acid eutectic crystal and preparation method thereof
A technology of famotidine and malic acid, which is applied in the field of co-crystal of famotidine and malic acid and its preparation, can solve the problem of only 40% to 50%, and achieve the effect of improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation of eutectic
[0032] Weigh 0.3g of famotidine and add it into 100ml of methanol and sonicate (power 200W) for 30 minutes, add 0.1g of malic acid and continue to sonicate for 1 hour, leave at room temperature to precipitate a white precipitate, filter and dry to obtain famotidine-malic acid cocrystal.
Embodiment 2
[0034] Weigh 0.3g of famotidine and add it to 100ml of methanol, heat (50-70°C) and stir for 30 minutes, add 0.1g of malic acid and continue heating and stirring for 1 hour, leave it at room temperature to precipitate a white precipitate, filter and dry to obtain famotidine-apple Acid eutectic.
Embodiment 3
[0036] Weigh 0.1 g of famotidine and add it to 100 ml of acetonitrile for 30 minutes of sonication, add 0.04 g of malic acid and continue sonication for 1 hour, leave at room temperature to precipitate a white precipitate, filter and dry to obtain famotidine-malic acid cocrystal.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com