Time-resolved fluorescence immunoassay kit for detecting novel coronavirus and preparation method thereof
A time-resolved fluorescence and coronavirus technology, applied in the analysis of materials, fluorescence/phosphorescence, biological material analysis, etc., can solve the problems of high requirements for detection equipment, poor repeatability, and many interference factors, and achieve convenient and specific detection operations Strong and sensitive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1. Preparation of Time-Resolved Fluorescence Immunochromatography Kit for Detecting Novel Coronavirus
[0044] Time-resolved fluorescent immunochromatography kit for the detection of novel coronavirus COVID-19. The test strip adopts the principle of double-antibody sandwich immunochromatography to detect the new coronavirus in human samples (respiratory tract, blood, cornea, stool, etc.) Content of the virus COVID-19. The kit consists of a time-resolved fluorescent immunochromatographic test strip for detecting the novel coronavirus COVID-19, a sample buffer and an ID card containing the standard curve of the novel coronavirus COVID-19.
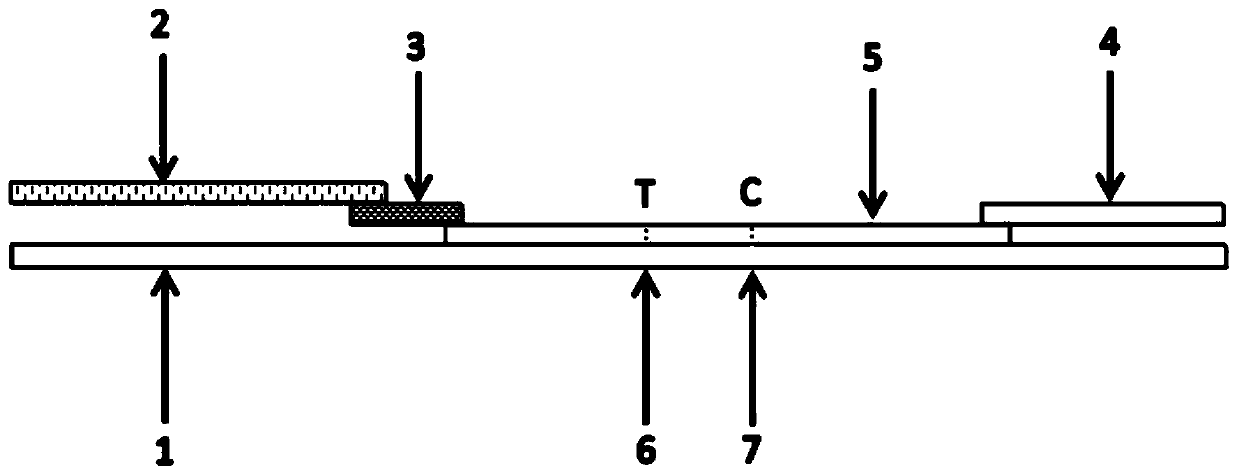
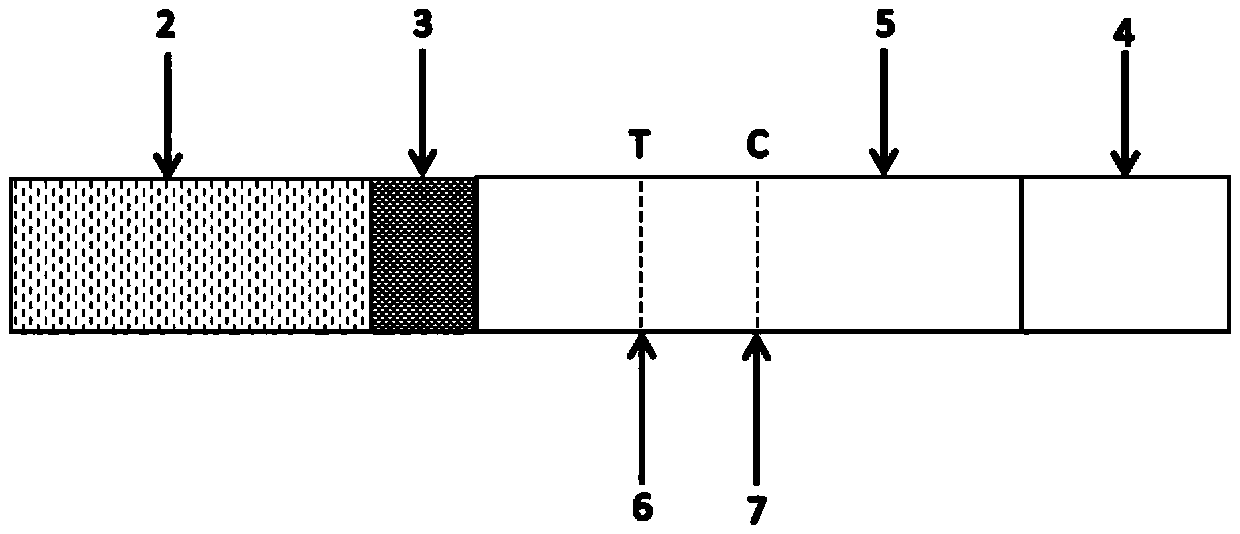
[0045] Specifically, the test strip of the present invention is as figure 1 and figure 2 As shown, the sample filter pad 2, microsphere marking pad 3, detection pad 5, and water-absorbing pad 4 are sequentially overlapped on the PVC bottom plate 1; The COVID-19 monoclonal antibody coating; the test pad 3 has a test line (T)...
Embodiment 2
[0059] Example 2, Time-resolved fluorescence immunochromatography kit for quantitative detection of novel coronavirus
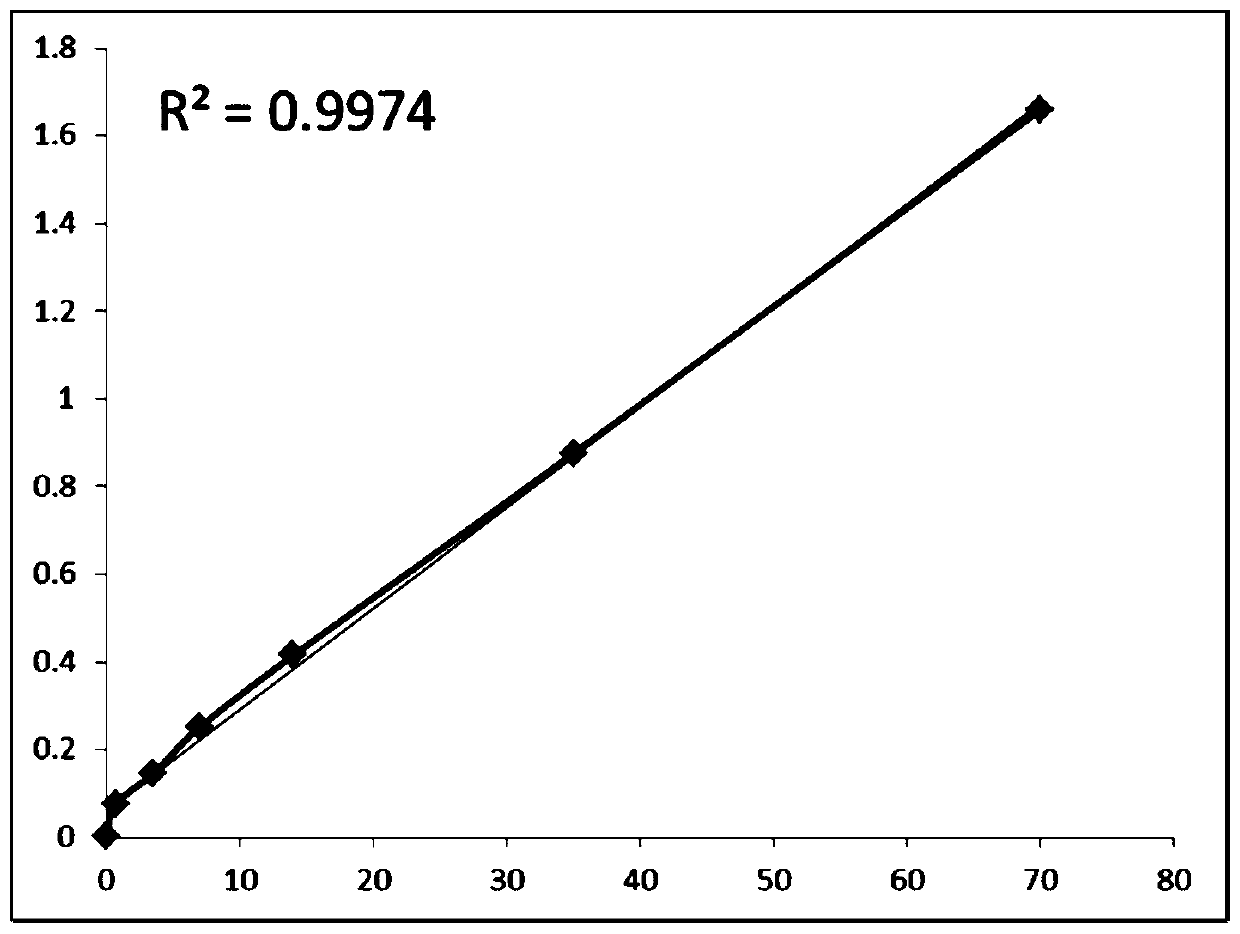
[0060] Add different concentrations of human novel coronavirus COVID-19 antigen calibrator (70ng / ml, 35ng / ml, 14ng / ml, 7ng / ml, 3.5ng / ml, 0.7ng / ml to the test strip prepared according to Example 1. ml, 0.0ng / ml), a total of seven concentrations, and each concentration set three repetitions, all of which were prepared by diluting 0.5mg / ml human novel coronavirus COVID-19N protein recombinant antigen with sample buffer, after 15min of chromatography, passed The dry fluorescent immunoassay analyzer reads the fluorescence intensity ratio of the detection line and the quality control line, wherein the fluorescence intensity ratio=detection value of the detection line / detection value of the quality control line.
[0061] Take the concentration of the new coronavirus COVID-19 calibrator as the X-axis, and take the fluorescence intensity of the sample detection line / q...
Embodiment 3
[0072] Example 3, Time-resolved fluorescence immunochromatography kit for qualitative detection of novel coronavirus
[0073] Prepare test strips according to Example 1 of the present invention, and set four different concentrations of human novel coronavirus COVID-19 antigen calibrator: 70ng / ml, 7ng / ml, 0.7ng / ml, 0.0ng / ml.
[0074] Follow the steps below to perform a qualitative test:
[0075] (1) Put the sample to be tested and the detection reagent to room temperature, and wait for the temperature to balance;
[0076] (2) Take 100 μl of the sample to be tested and add it to 100 μl of sample buffer, mix well to obtain a mixed sample;
[0077] (3) Take 100 μl of the above-mentioned mixed sample, add it to the sample hole of the test strip, and react at room temperature for 10-15 minutes in the dark;
[0078] (4) Turn on the portable ultraviolet detection pen, irradiate the detection test strip through the exposed part of the detection window, observe the fluorescent display...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com