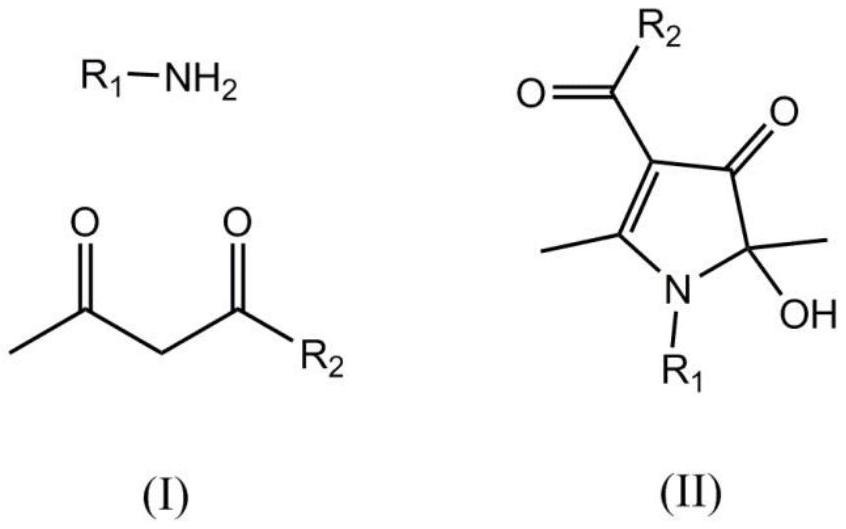
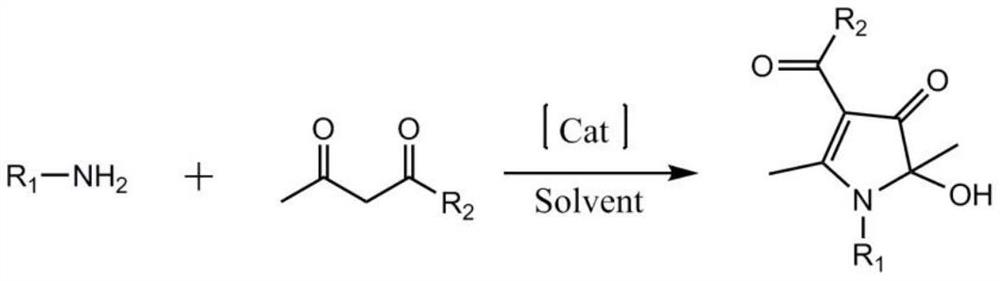
A kind of synthetic method of 1h-3-pyrrolidone compound
A technology of pyrrolidone and synthesis method, which is applied in chemical instruments and methods, organic compound/hydride/coordination complex catalyst, chemical/physical process, etc., can solve the problem of increased cost, single structure of pyrrolidone, and low reaction rate. It is difficult to control the amplification of heat and other problems, so as to achieve the effect of simple production process, low emission and mild production process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
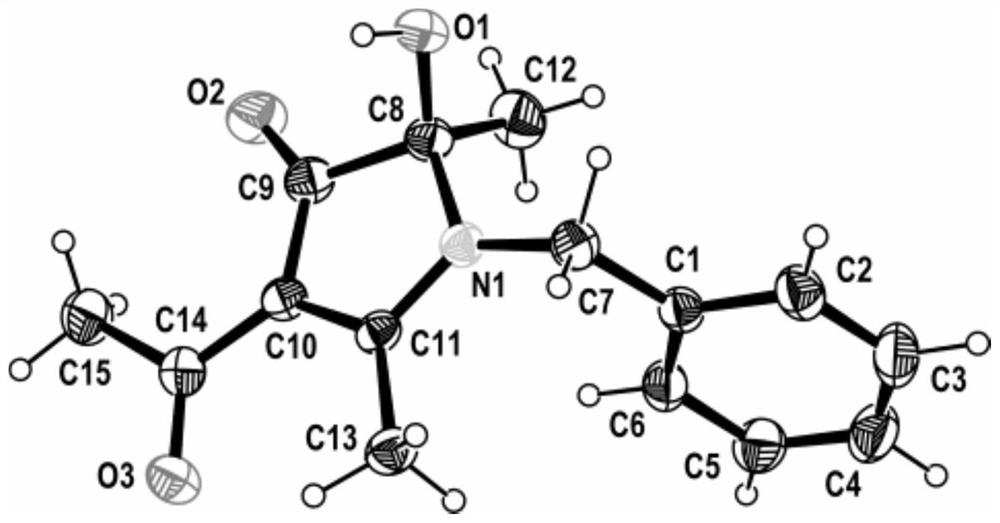
[0023] In a 250ml round-bottom flask, add acetylacetone (22.0g, 220mmol), benzylamine (10.7g, 100mmol), the complex formed by ferric chloride and 1,10-phenanthroline (3.0mmol), cuprous iodide ( 1.5mmol) and toluene (100ml). Turn on stirring and raise the temperature, and react at 105°C (105°C±2°C) for 8 hours, during which the bottle mouth is kept open. The temperature was stopped, and then 100 ml of deionized water was added. After the two phases were separated, the aqueous phase was extracted three times with ethyl acetate, and the organic phases were combined and dried over anhydrous sodium sulfate. After filtration, distillation under reduced pressure, the concentrate is separated by chromatographic column (the column filler is silica gel, and the elution is calculated as the ratio of petroleum ether and ethyl acetate is 5:1) or recrystallization separation (mixed solvent of acetone and ethyl acetate) , 21.1 g of colorless crystals were obtained, the yield was 85%, the pr...
Embodiment 2
[0025] In a 250ml round-bottomed flask, add acetylacetone (22.0g, 220mmol), n-butylamine (7.4g, 100mmol), the complex formed by iron nitrate and L-lysine (3.0mmol), cuprous iodide (1.5mmol) ) and toluene (100 ml). Turn on stirring and raise the temperature, and react at 105°C (105°C±2°C) for 8 hours, during which the bottle mouth is kept open. The temperature was stopped, and then 100 ml of deionized water was added. After the two phases were separated, the aqueous phase was extracted three times with ethyl acetate, and the organic phases were combined and dried over anhydrous sodium sulfate. After filtration, distillation under reduced pressure, the concentrate is separated by chromatographic column (the column filler is silica gel, and the elution is calculated as the ratio of petroleum ether and ethyl acetate is 5:1) or recrystallization separation (mixed solvent of acetone and ethyl acetate) , 15.1g of colorless crystals were obtained, the yield was 67%, the product purit...
Embodiment 3
[0027] In a 250ml round-bottomed flask, add acetylacetone (22.0g, 220mmol), n-butylamine (7.4g, 100mmol), the complex formed by ferric chloride and L-proline (3.0mmol), and copper oxide (1.5mmol) and DMSO (100ml). Turn on stirring and raise the temperature, and react at 105°C (105°C±2°C) for 8 hours, during which the bottle mouth is kept open. The temperature was stopped, and then 100 ml of deionized water was added. After the two phases were separated, the aqueous phase was extracted three times with ethyl acetate, and the organic phases were combined and dried over anhydrous sodium sulfate. After filtration, distillation under reduced pressure, the concentrate is separated by chromatographic column (the column filler is silica gel, and the elution is calculated as the ratio of petroleum ether and ethyl acetate is 5:1) or recrystallization separation (mixed solvent of acetone and ethyl acetate) , 15.1g of colorless crystals were obtained, the yield was 67%, the product purit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com