Itopride hydrochloride tablet and preparation method of tablet
A technology of itopride hydrochloride tablets and itopride hydrochloride, which is applied in the direction of pharmaceutical formula, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of water becoming sticky and easy to absorb moisture , material stickiness and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Preparation of Itopride Hydrochloride Tablets (Specification: 0.05g)
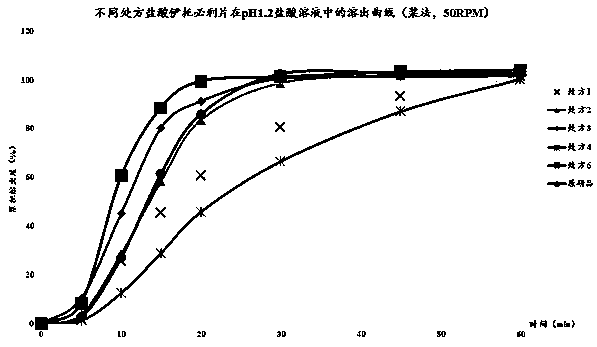
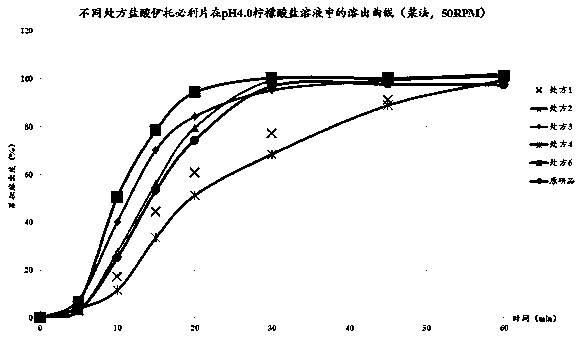
[0032]As shown in Table 1, according to the type of material designed by the prescription, the bulk drug and auxiliary materials are vibrated through a 60-mesh sieve. The excipients include fillers (lactose, corn starch), disintegrants (carboxymethylcellulose), glidants (silicon dioxide), lubricants (magnesium stearate). Weigh the crude drug, filler, disintegrant, glidant and lubricant according to the prescription design, place in a three-dimensional mixer, mix for 15 minutes, and detect the actual content of itopride hydrochloride. Put it in the feeding hopper of a Bosch tablet press, the design tablet weight is 130mg, the tablet hardness is about 50-80N, and the tablet is pressed. The particle size distribution of the raw material is D10<10μm, D50<20μm, D90<50μm, the tableting speed is 300,000 tablets / hour, and the HPMC coating material of Colorcon is used for coating, and the weight ga...
Embodiment 2
[0038] Example 2 Comparison of Accelerated Test Stability Study of Prescription 2 Itopride Hydrochloride Tablets and Original Research Product (R)
[0039] Take itopride hydrochloride tablets and the original research product R, pack them in aluminum-plastic blisters, and place them in a constant temperature and humidity box with a temperature of 40±2°C and a humidity of RH75%±5%. Sampling once at the end of 1 month and 6 months respectively to check its properties, content, dissolution rate and related substances, the results are shown in Table 6.
[0040]
[0041] The results in Table 6 show that the itopride hydrochloride tablets prepared by prescription 2 of the present invention are placed at 40 ± 2°C for 3 months and 6 months respectively in a constant temperature and humidity chamber with humidity RH75% ± 5%. , dissolution rate and content have no significant change, showing that the itopride hydrochloride tablet prepared by the present invention has good stability a...
Embodiment 3
[0042] Example 3 Comparison of Pharmacokinetic Study of Prescription 2 Itopride Hydrochloride Tablets and Original Research Product (R) in Human Body
[0043] The purpose of this test is to evaluate the oral administration of itopride hydrochloride tablets (specification: 0.05g, prescription 2, test preparation) and the original research product of itopride hydrochloride tablets (trade name: Weilisu; specification : 0.05g; Licensee: ABBOTT LABORATORIES (M) SDN.BHD) after the pharmacokinetic characteristics and bioequivalence. Once the subjects were selected, 12 subjects were randomly divided into 3 groups of 4 persons.
[0044] At about 8:00 on the first day of the study, the subjects ate a high-fat meal (providing about 50% of the calories in the food), a high-calorie meal (about 800-1000 kcal, of which protein provided about 150 kcal and carbohydrates about Provide 250 kcal of heat, fat provides about 500-600 kcal of heat), eat within 30 minutes. 30 minutes after the subje...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com