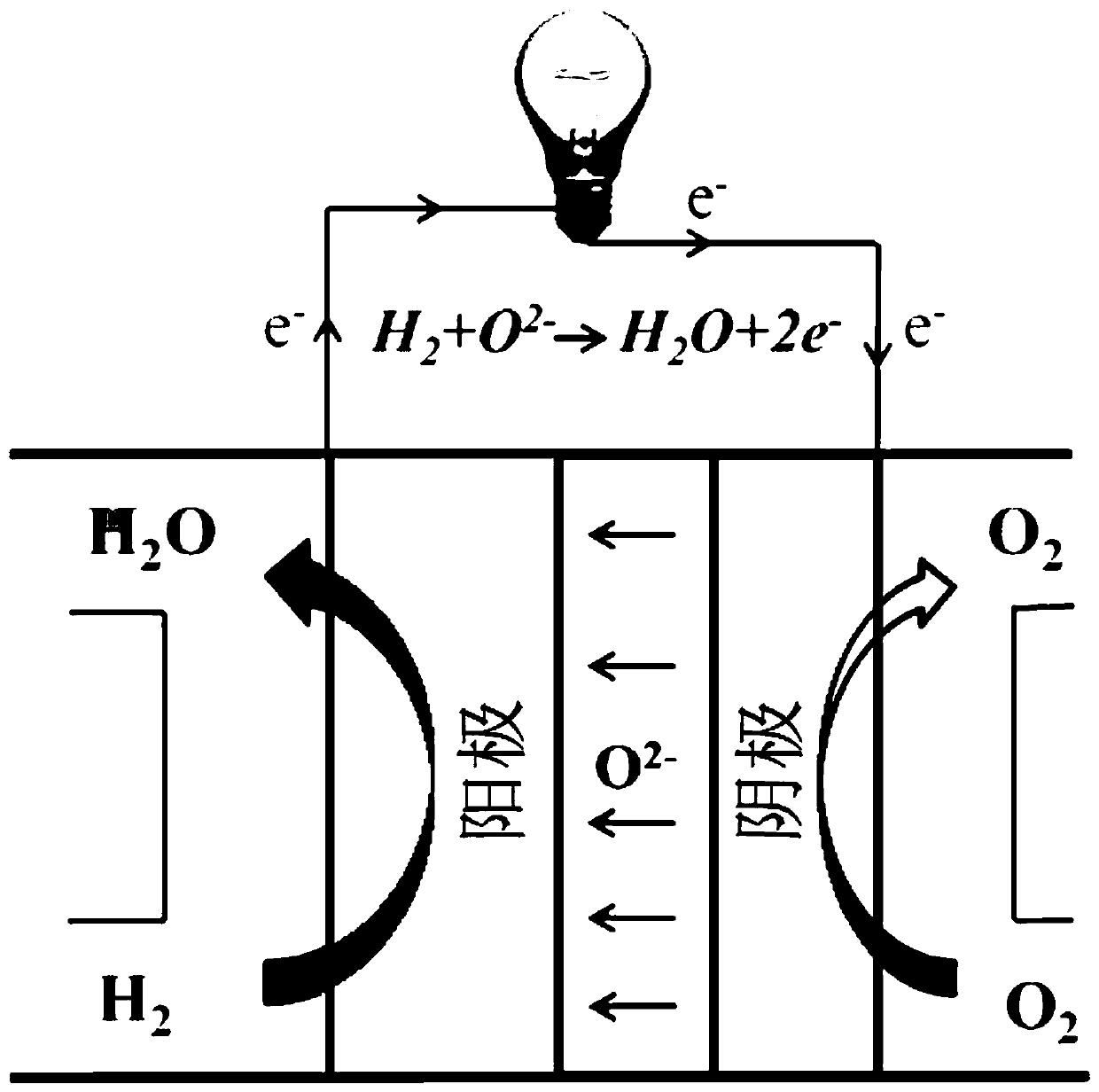
Oxide material of iron-based double perovskite structure and preparation method thereof
A double perovskite and oxide technology, applied in the direction of fuel cells, electrochemical generators, final product manufacturing, etc., can solve the problems of high thermal expansion coefficient, high cost of cobalt raw materials, and limited practical applications, etc., to achieve high ion and electronic The effect of electrical conductivity, reduced sintering temperature, and good anti-carbon deposition effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] This embodiment provides an oxide material GdBaFe of a first configuration iron-based double perovskite structure 2 o 5+δ , which was prepared by the following steps:
[0066] (1) Weigh Gd according to the molar ratio Gd:Ba:Fe=1:1:2 2 o 3 , Ba(NO 3 ) 2 and Fe(NO 3 ) 3 9H 2 O, where Gd 2 o 3 Pre-burn at 900°C for 2 hours to remove moisture, and weigh the required amount at 200°C; weigh the obtained Gd 2 o 3 Dissolved in dilute nitric acid to obtain the corresponding nitrate solution, and then mixed with Ba(NO 3 ) 2 and Fe(NO 3 )3 9H 2 The aqueous solution of O was mixed to obtain a mixed solution of three nitrates (referred to as the first mixed solution in Example 1).
[0067] (2) Take complexing agent ethylenediaminetetraacetic acid and citric acid, wherein, the mol ratio of ethylenediaminetetraacetic acid, citric acid and metal ion is 0.7:1.8:0.9, and metal ion refers to Gd 3+ 、Ba 2+ , Fe 3+ The sum of the three is mixed with the above-mentioned firs...
Embodiment 2
[0075] This embodiment provides an oxide material GdBaFe of a first configuration iron-based double perovskite structure 2 o 5+δ , which was prepared by the following steps:
[0076] (1) Weigh Gd according to the molar ratio Gd:Ba:Fe=1:1:2 2 o 3 , Ba(NO 3 ) 2 and Fe(NO 3 ) 3 9H 2 O, where Gd 2 o 3 Pre-burn at 900°C for 2 hours to remove moisture, and weigh the required amount at 200°C. Will weigh the resulting Gd 2 o 3 Dissolve in dilute nitric acid to obtain the corresponding nitrate solution. Then with Ba(NO 3 ) 2 and Fe(NO 3 ) 3 9H 2 The aqueous solution of O was mixed to obtain a mixed solution of three nitrates (referred to as the first mixed solution in Example 2).
[0077] (2) Take complexing agent ethylenediaminetetraacetic acid and citric acid, wherein, the mol ratio of ethylenediaminetetraacetic acid, citric acid and metal ion is 0.9:1.9:1.1, and metal ion refers to Gd 3+ 、Ba 2+ , Fe 3+ The sum of the three. After adding ammonia water to dissolv...
Embodiment 3
[0084] This embodiment provides a second configuration of the iron-based double perovskite structure oxide material GdBaFe 2 o 5+δ , which was prepared by the following steps:
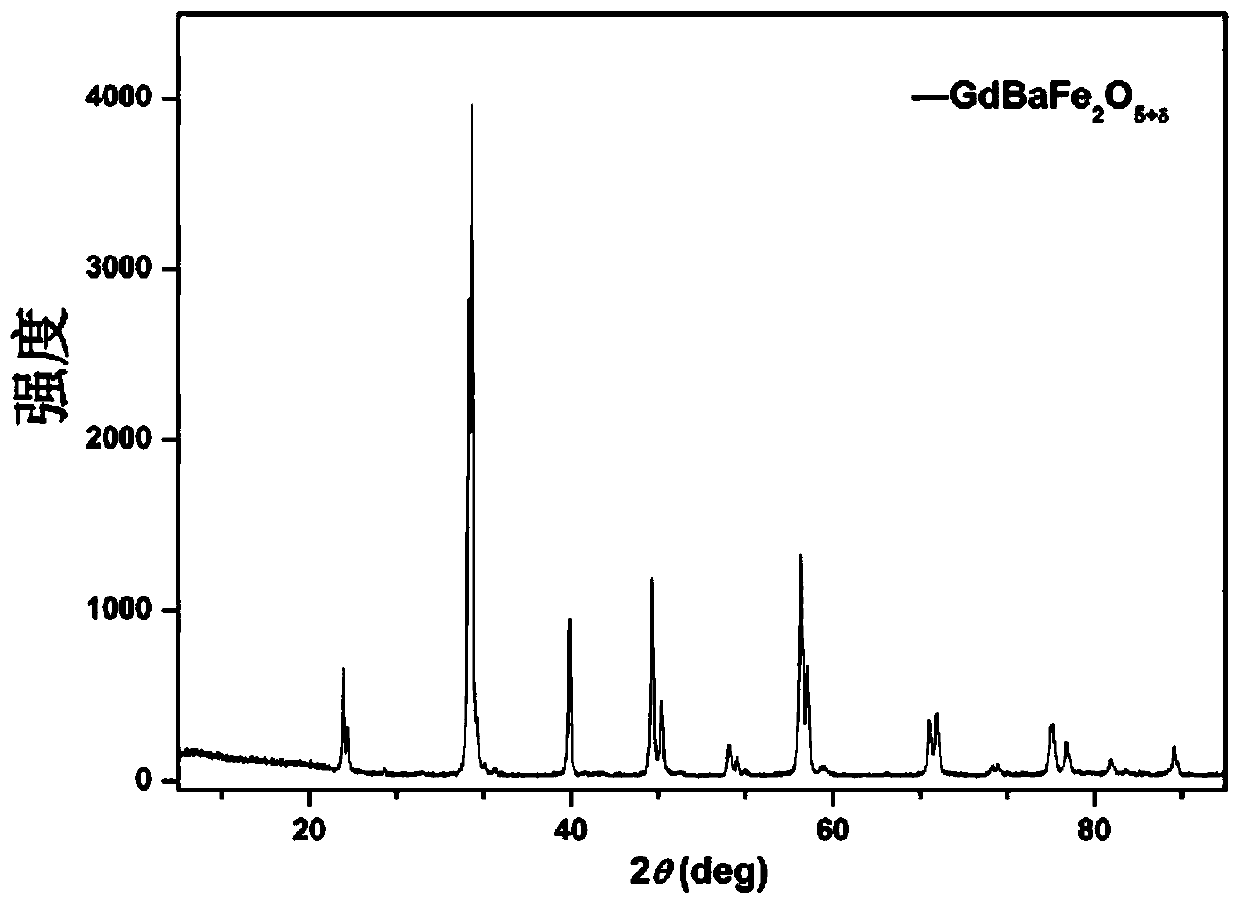
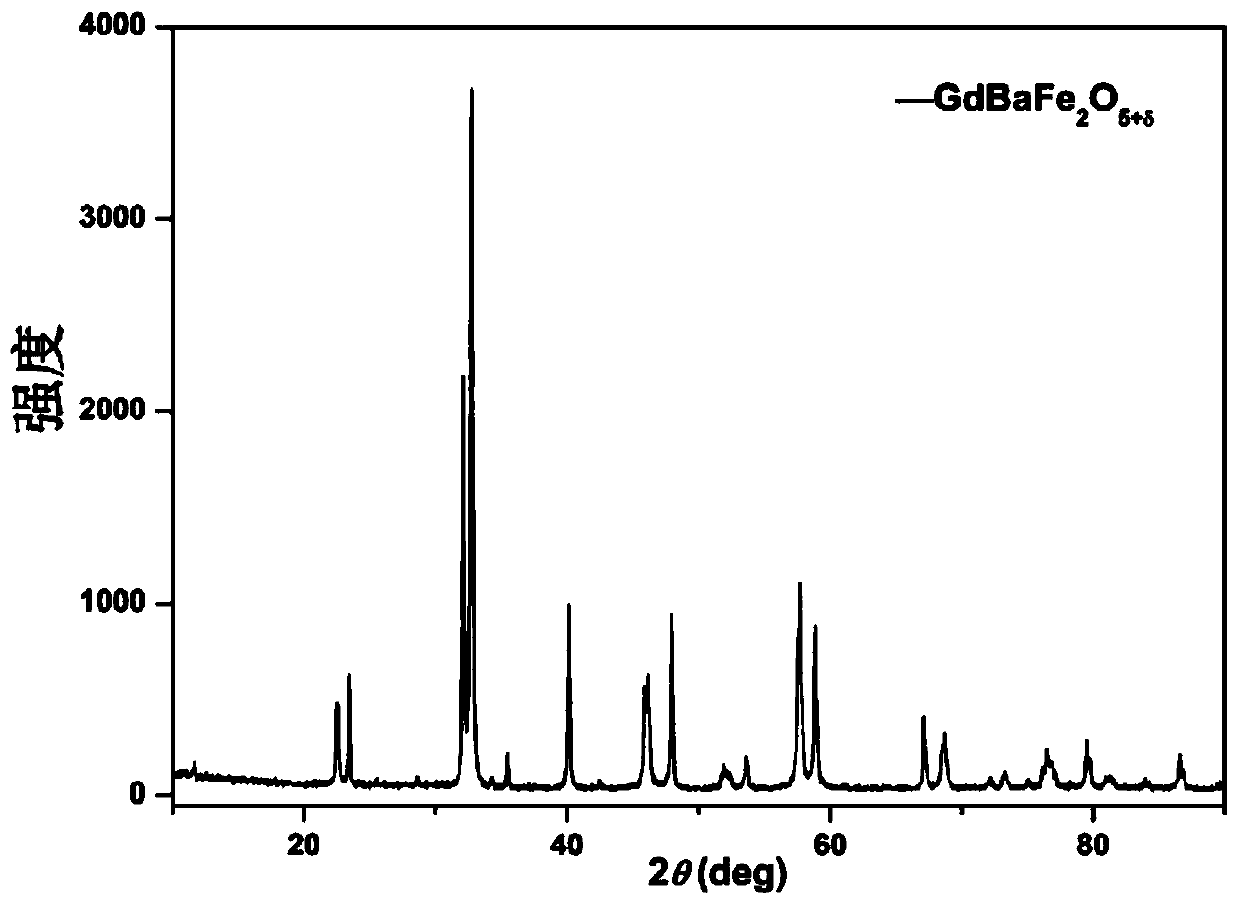
[0085] The GdBaFe prepared by embodiment 1 2 o 5+δ After grinding with alcohol for 1 hour, sintering at 1000°C for 15 hours in a reducing atmosphere to obtain the iron-based double perovskite structure oxide material GdBaFe that can be used as the second configuration of the solid oxide fuel cell anode material 2 o 5+δ , wherein the reducing atmosphere is 3% hydrogen and 97% argon at normal pressure.
[0086] image 3 For the prepared GdBaFe of this embodiment 2 o 5+δ Compared with the standard card in the crystal library, it can be seen that there are no other impurity peaks, indicating that after high temperature sintering, a single-phase iron-based double perovskite structure oxide GdBaFe was obtained under a reducing atmosphere. 2 o 5+δ , and an obvious double-peak splitting phenomenon app...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average coefficient of thermal expansion | aaaaa | aaaaa |
| Average coefficient of thermal expansion | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com