Production of isoquercetin derivative used for scavenging free radicals
A technology of isoquercetin and its derivatives, which is applied in the field of preparation of isoquercetin derivatives, can solve the problems of inconspicuous effect, low solubility of isoquercetin, and low bioavailability of isoquercetin, and increase the Efficacy, increased solubility, improved bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Preparation of 6-prenyl substituted isoquercetin derivatives
[0043] 1) React 2 g of isoquercetin, 3-4 equivalents of acetic anhydride, and 1-2 equivalents of pyridine at 100-140° C. for 15 hours, and the product is IIIa with a yield of 90.5%;
[0044] 2) 2 g of IIIa, 3 times of thiophenol, 0.1-1 times of imidazole, and N-methylpyrrolidone as a solvent were reacted at 0°C for 4-20 hours, and the product was IIIb with a yield of 85.1%;
[0045] 3) React Ⅲb with 2-4 times (2-methylbut-3-en-2-yl) isobutyl carbonate, triphenylphosphine as catalyst, in THF solution at -5-0°C for 12h to obtain Ⅲc The yield is 92.1%;
[0046] 4) 2 g of IIIc was added with 2 times equivalent of acetic anhydride and 10 times of acetic anhydride, and refluxed in methanol for 24 hours to obtain product IIId with a yield of 88.0%.
[0047]
Embodiment 2
[0048] Example 2 Glycosylation of prenylated isoquercetin derivatives
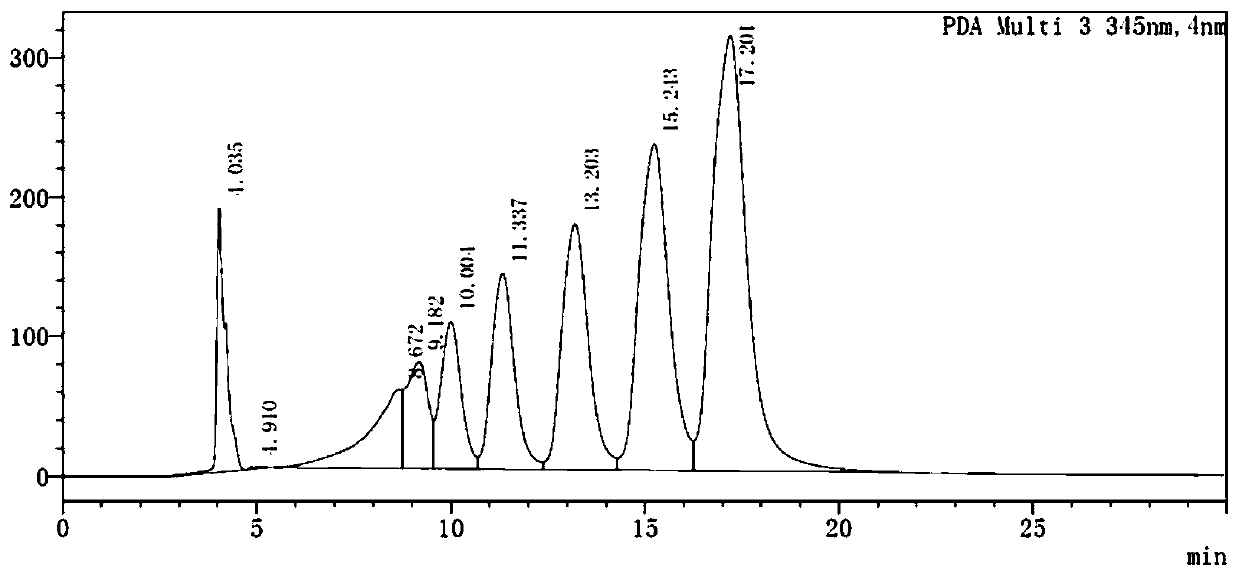
[0049] Using 6-prenyl substituted isoquercetin derivatives as raw materials, the reaction buffer is disodium hydrogen phosphate-sodium citrate buffer with a mass fraction of 0.05%, the pH of the buffer is 8, and the reaction temperature is 65°C , The reaction time is controlled for 18-24 hours. Glucosyltransferase is added into the reaction system, and the mass fraction of the enzyme is 25-30% of the input 6-prenyl substituted isoquercetin derivative to obtain the isoquercetin derivative. In the reaction system, the molar yield of the isoquercetin derivative is 95.8%. After the reaction is completed, the isoquercetin derivative is obtained by suction filtration and drying. HPLC of isoquercetin derivatives see image 3 .
Embodiment 3
[0050] Solubility and dissolution rate determination of embodiment 3 isoquercetin derivatives
[0051] 1. Determination of Solubility
[0052] The solubility of the compound directly affects the application of the drug in the solution system and cell system. Because isoquercetin derivatives are stable in aqueous solution, we use ultraviolet spectrophotometry to determine the solubility value of saturated isoquercetin aqueous solution. In this experiment, 10mg, 50mg, 100mg, 200mg, and 1000mg of phloretin were accurately measured, placed in a 100mL volumetric flask, diluted to the mark with DMSO, and shaken to obtain a series of concentrations of 0.1mg / mL, 0.5mg / mL, 1mg / mL, 2mg / mL, 10mg / mL standard solutions of phloretin were analyzed by HPLC, the characteristic peaks in the 283nm interval were integrated, and the peak areas were recorded. Take the concentration of phloretin as the ordinate, and the peak area as the abscissa to draw a graph, and perform linear regression.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com