Diphenolic acid type polyarylether dielectric material with side chain containing methylsulfonyl and preparation method thereof
A technology of bisphenolic acid type and dielectric material is applied in the field of bisphenolic acid type polyarylene ether dielectric material and its preparation to achieve the effects of increasing orientation polarization, easy realization of preparation conditions and increasing polarizability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
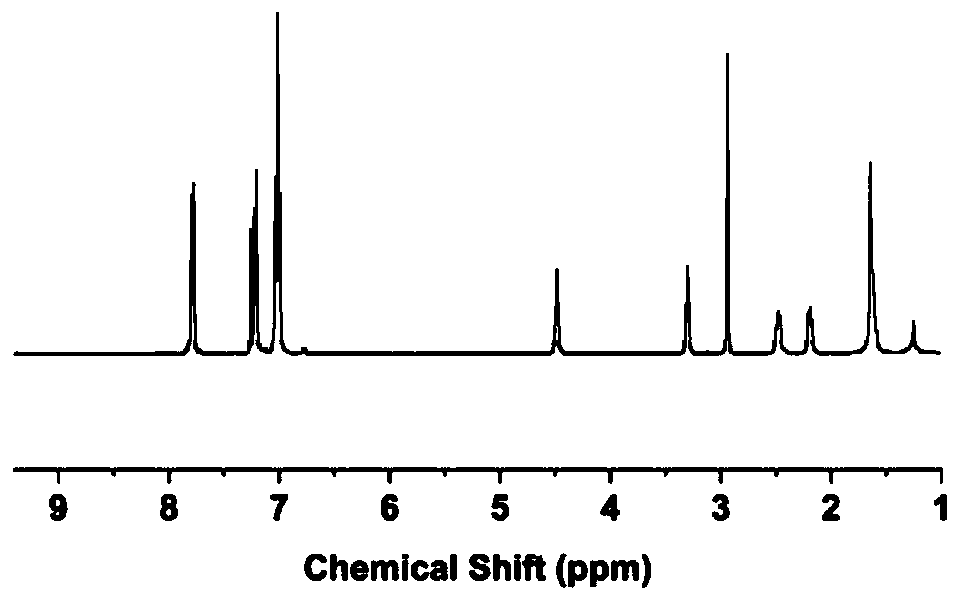
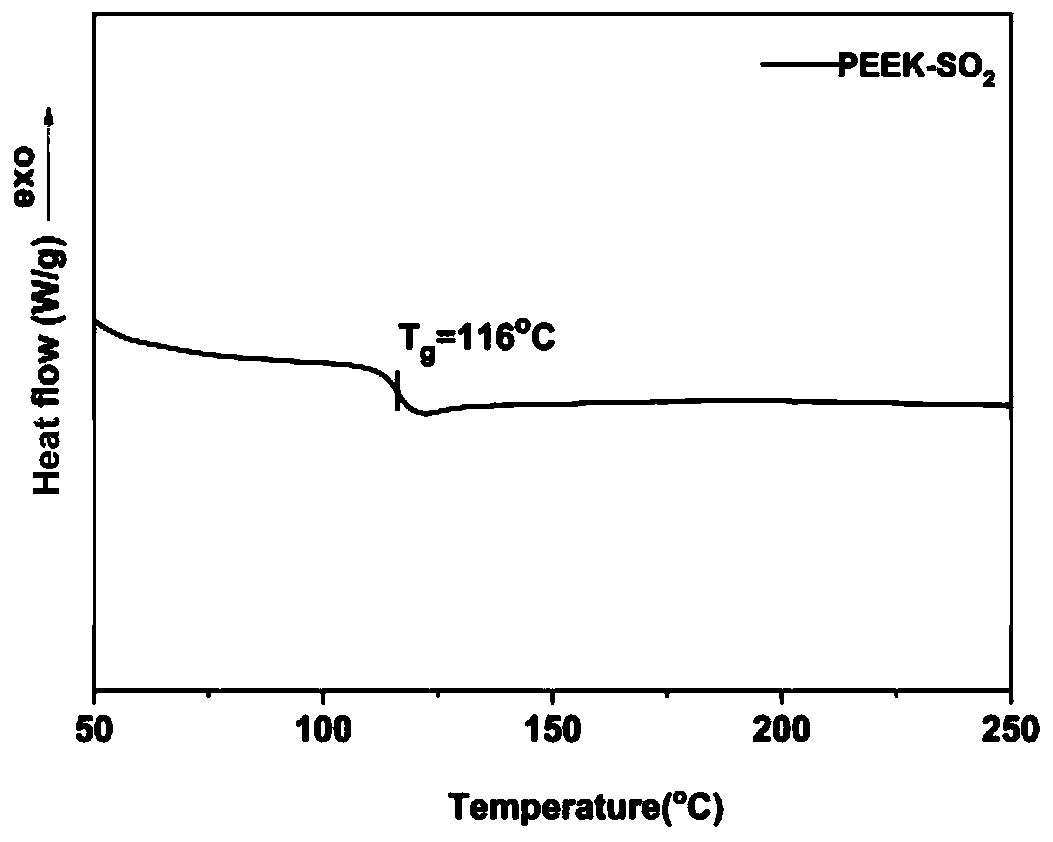
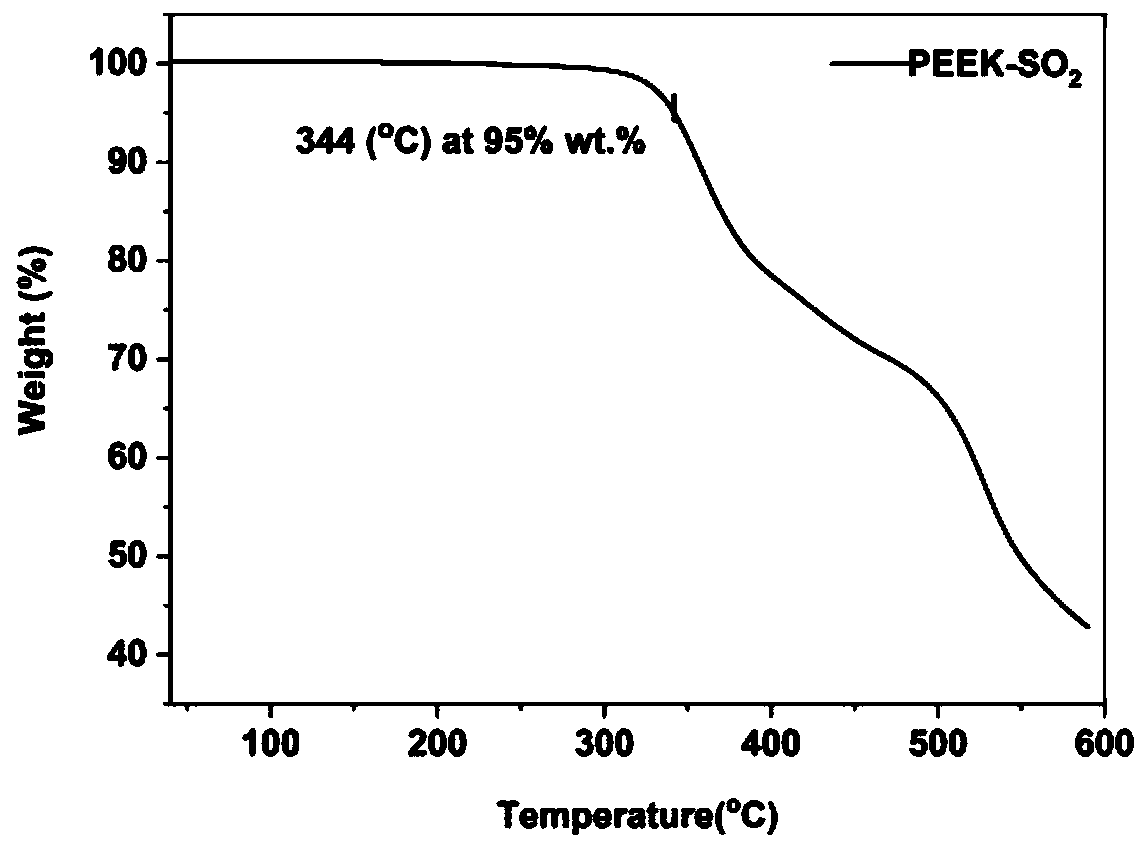
[0044] Example 1: PEEK-SO 2 Synthesis,
[0045] Step 1, put bisphenolic acid, potassium carbonate, and potassium hydroxide into a reactor with a condensing tube in a molar ratio of 1:1.4:0.3, and add toluene and N-formaldehyde according to a volume ratio of 1:3 1-pyrrolidone, under the protection of nitrogen, reflux at 120°C for 1 hour, cool to room temperature, then add 4,4'-difluorobenzophenone, reflux at 140°C for 2 hours, remove the water and toluene in the reaction system, at 168°C Lower reaction 5h;
[0046] Step 2, the product of step 1 is poured into acetone while it is hot, soaked in hydrochloric acid for 24 hours, filtered and dried, and then dissolved in N,N-dimethylformamide, wherein the product is mixed with N,N-dimethyl The ratio of formamide is 1g:8mL, the solution is added dropwise to deionized water to precipitate, after three times of washing, suction filtration and drying, PEEK-COOH is obtained;
[0047] Step 3, weigh 2 g of purified PEEK-COOH and place i...
Embodiment 2
[0057] Example 2: PAEN-SO 2 Synthesis,
[0058] Step 1, put bisphenolic acid, potassium carbonate, and potassium hydroxide into a reactor with a condensing tube in a molar ratio of 1:1.5:0.4, and add toluene and N-formaldehyde in a volume ratio of 1:3 Base pyrrolidone, under the protection of nitrogen, reflux at 130°C for 2 hours, cool to room temperature, then add 2,6-dichlorobenzonitrile, reflux at 150°C for 3 hours, remove the water and toluene in the reaction system, and react at 175°C 6h;
[0059] Step 2, the product of step 1 is poured into acetone while it is hot, soaked in hydrochloric acid for 24 hours, filtered and dried, and then dissolved in N,N-dimethylformamide, wherein the product is mixed with N,N-dimethyl The ratio of formamide is 1g:8mL, the solution is added dropwise to deionized water to precipitate, after 5 times of washing, suction filtration and drying to obtain PAEN-COOH;
[0060] Step 3, weigh 2g of purified PAEN-COOH and place it in a round bottom ...
Embodiment 3
[0067] Example 3: PAES-SO 2 Synthesis,
[0068] Step 1, put bisphenolic acid, potassium carbonate, and potassium hydroxide into a reactor with a condensing tube in a molar ratio of 1:1.6:0.4, and add toluene and N-formaldehyde in a volume ratio of 1:3 Under the protection of nitrogen, reflux at 140°C for 3 hours, cool to room temperature, then add 4,4'-difluorodiphenyl sulfone, reflux at 160°C for 5 hours, remove the water and toluene in the reaction system, at 185°C Reaction 7h;
[0069] Step 2, the product of step 1 is poured into acetone while it is hot, soaked in hydrochloric acid for 24 hours, filtered and dried, and then dissolved in N,N-dimethylformamide, wherein the product is mixed with N,N-dimethyl The ratio of formamide is 1g: 8mL, the solution is added dropwise to deionized water to precipitate, and after 4 times of washing, suction filtration and drying, PAES-COOH is obtained;
[0070] Step 3, weigh 2 g of the purified PAES-COOH and place it in a round bottom f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com