Application of salvianolic acid a in the preparation of medicines for the treatment of pneumonia caused by methicillin-resistant Staphylococcus aureus
A staphylococcus infection, methicillin-resistant technology, applied in the field of medicine, can solve problems such as treatment failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
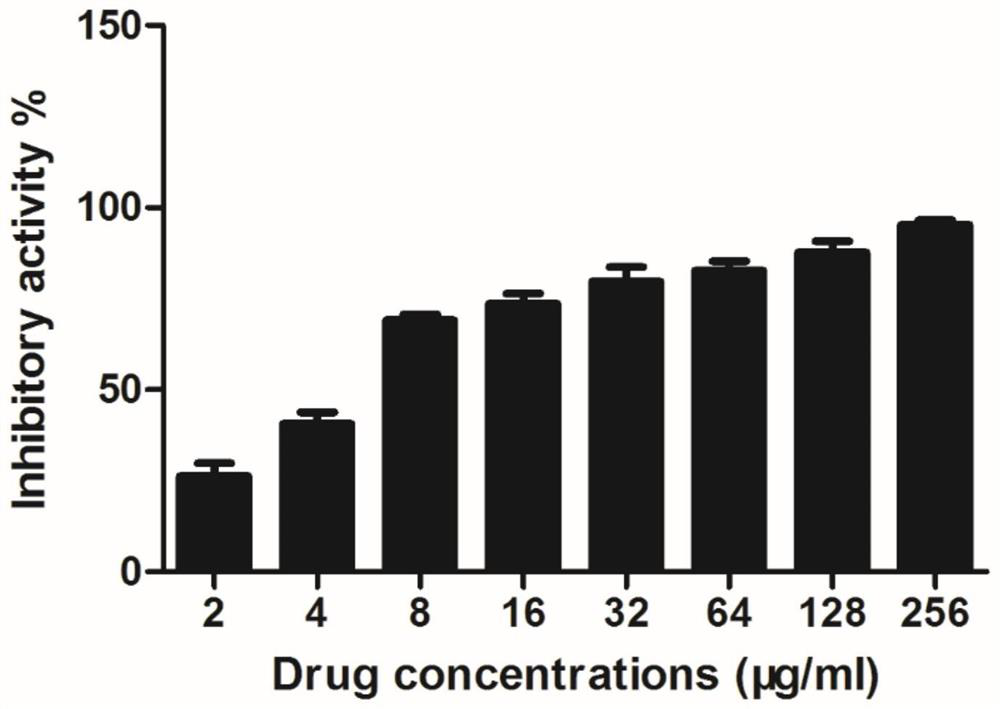
[0025] Embodiment 1, the inhibitory effect of salvianolic acid A on Staphylococcus aureus virulence factor SrtA
[0026] Inoculate Escherichia coli BL21, an expression strain containing the pET28a-SrtA vector, into LB medium, shake at 200 rpm overnight at 37°C. On the next day, re-inoculate into LB medium with Kanna resistance at a ratio of 1:100, culture at 37°C, 200rpm to OD 600 Up to 0.6-0.8. Take 1 mL of bacterial liquid as the pre-induction control. For the remaining 30 min on ice, IPTG with a final concentration of 1 mM was added to induce overnight at 16°C and 180 rpm. On the next day, 1 mL of the bacterial liquid was taken as a post-induction control, and the bacterial cells were collected by centrifugation (4000 rpm, 30 min). The collected bacterial cells were resuspended with protein buffer, ultrasonically disrupted by using a sonicator, and the crushed supernatant and precipitate were collected by a centrifuge (12000 rpm, 1 h). Finally, the expression of protein...
Embodiment 2
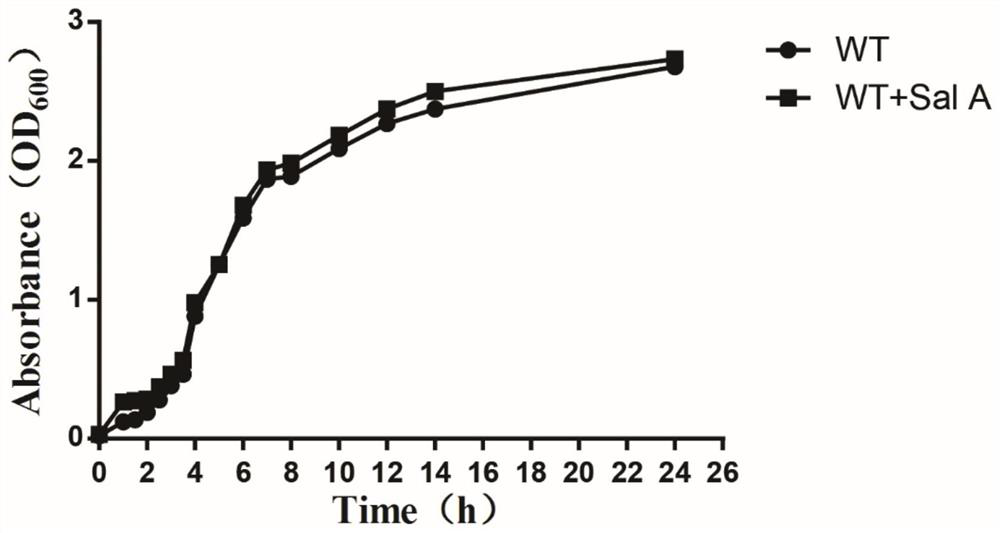
[0029] Embodiment 2, the influence of salvianolic acid A on the growth of Staphylococcus aureus USA300
[0030] In order to further confirm that salvianolic acid A has no effect on the growth of Staphylococcus aureus USA300, the growth of Staphylococcus aureus USA300 in the presence of 256 μg / mL salvianolic acid A was measured. Staphylococcus aureus USA300 cultured overnight was added to sterile BHI broth containing or not containing 256 μg / mL of traditional Chinese medicine monomer at a ratio of 1:100, and grown at 37°C for 24 hours with shaking. The DMSO group was used as a control. The absorbance at 600 nm was measured at different time intervals using a UV spectrophotometer, and the growth curve was drawn. The results are attached figure 2 shown. Even though the dose of salvianolic acid A is its IC 50 40 times that of S. aureus, the growth rate of S. aureus was still similar to that of WT. The results showed that salvianolic acid A could be used as a potential antivi...
Embodiment 3
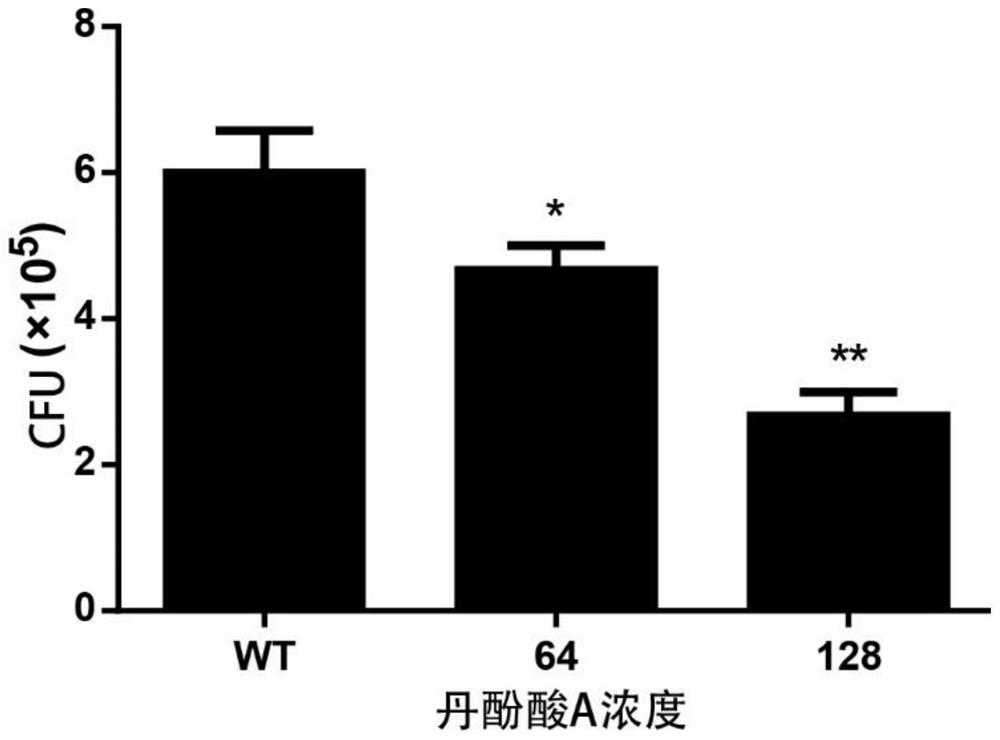
[0031] Example 3, Salvianolic acid A inhibits Staphylococcus aureus from invading A549 cells
[0032] Human lung epithelial cells A549 were suspended in DMEM / high glucose medium (containing 10% fetal bovine serum), and then cultured 3×10 5 Cells were inoculated into 24-well cell culture plates (cover slips with a diameter of 12 mm), placed in a cell culture incubator at 37 ° C, 5% CO2 Incubate overnight. The Staphylococcus aureus cultured overnight was inoculated into the medium containing salvianolic acid A at a final concentration of 64 μg / mL and 128 μg / mL at a ratio of 1:100, and the culture was continued until the absorbance was OD 600nm = 1.0 for later use. Take out 1mL Staphylococcus aureus suspension from each group (absorbance is OD 600nm =1.0), added to the corresponding cells in the 24-well cell culture plate, with 3 replicates in each group, at 37°C, 5% CO 2 Incubate for 60 minutes under conditions. Each well was washed 3 times with PBS, then 1 mL of DMEM / high g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com