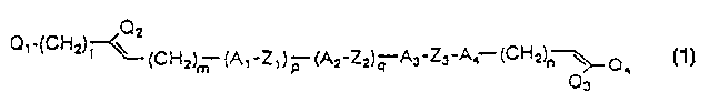Bisalkenyl derivatives, liquid crystalline compound and liquid crystal composition
A liquid crystal compound and alkenyl technology, which is applied in the preparation of carbon-based compounds, silicon organic compounds, organic compounds, etc., can solve problems such as narrow temperature range, poor solubility, and poor appearance of liquid crystal phases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1V2-HH-VFF(No.25) 30.0%V-BHH-VFF(No.7) 19.0%V-BBH-VFF(No.9) 15.0%3-HB-C 5.0%1V2-BEB(F,F)-C 11.0%3-HB-02 4.0%3-HB(F)TB-2 4.0%3-H2BTB-2 4.0%3-H2BTB-3 4.0%3-H2BTB-4 4.0% Embodiment 2V2-HH-VFF(No.25) 20.0%1V-BHH-VFF(No.19) 10.0%V-BBH-VFF(No.9) 6.0%3-HB-C 10.0%1V2-BEB(F,F)-C 12.0%3-HB-02 5.0%3-HHB-1 10.0%3-HHB-3 10.0%3-HB(F)TB-2 5.0%3-H2BTB-2 4.0%3-H2BTB-3 4.0%3-H2BTB-4 4.0% Embodiment 31V-BHH-VFF(No.19) 4.0%1V2-BHH-VFF(No.118) 10.0%V2-HB-C 10.0%1V2-HB-C 10.0%3-HB-C 26.0%5-HB-C 12.0%3-HB(F)-C 8.0%2-BEB-C 3.0%V2-HHB-1 8.0%3-H2BTB-2 3.0%3-H2BTB-3 3.0%3-H2BTB-4 3.0% Embodiment 4V2-HH-VFF(No.25) 9.0%1V2-BEB(F,F)-C 11.0%201-BEB(F)-C 5.0%301-BEB(F)-C 9.0%3-HB(F)-C 15.0%101-HH-3 3.0%4-BTB-02 9.0%2-HHB(F)-C 13.0%3-HHB(F)-C 14.0%3-H2BTB-2 4.0%3-H2BTB-3 4.0%3-H2BTB-4 4.0% Embodiment 51V2-BHH-VFF(No.118) 5.0%V-BHH-2VFF(No.8) 10.0%V2-HHH-VFF(No.29) 5.0%2-BB-C 5.0%2020-BB-C 4.0%101-HB-C 10.0%201-HB-C 7.0%2-BEB-C 12.0%5-PyB-F 8.0%2-PyB-2 2.0%3-PyB-2 2.0%4-PyB-2 2.0%V-HHB-1 5.0%2-PyBH-3 5.0%3-PyBH-3 5.0%4-PyBH-3 5.0%3-PyBB-F 3.0%4-PyBB-F 3.0%6-PyBB-2 2.0% Embodiment 6V2-HH-VFF(No.25) 3.0%3-PyB(F)-F 6.0%3020-BEB-C 4.0%3-BEB-C 12.0%3-DB-C 10.0%4-DB-C 10.0%3-HEB-04 8.0%4-HEB-02 6.0%5-HEB-01 6.0%3-HEB-02 5.0%5-HEB-02 4.0%3-HHB-1 6.0%3-HHEBB-C 3.0%3-HBEBB-C 3.0%10-BEB-2 5.0%4-HEB-3 3.0%5-HEB-1 3.0%6-PyB-02 3.0% Embodiment 7V2-HH-VFF(No.25) 4.0%1V-BHH-VFF(No.19) 3.0%3-HB-C 20.0%3-HHB-1 7.0%3-HHB-3 8.0%5-HEB-F 3.0%7-HEB-F 3.0%3-HHEB-F 1.0%5-HHEB-F 1.0%3-HEB-04 4.0%4-HEB-02 3.0%5-HEB-04 3.0%3-HEB-02 2.5%5-HEB-02 2.0%3-HB(F)TB-2 4.0%3-HB(F)TB-3 4.0%3-HB(F)VB-4 3.0%3-H2BTB-2 3.0%3-H2BTB-3 3.0% 3-H2BTB-4 3.0%5-HHEBB-C 2.0%3-HBEBB-C 3.0%5-HBEBB-C 3.0%3-HEBEB-F 3.0%3-HH-EMe 2.5%101-HBBH-3 2.0% Embodiment 8V-BHH-2VFF(No.8) 6.0%5-PyB(F)-F 13.0%2-HB(F)-C 10.0%3-HB(F)-C 12.0%30-BB-C 8.0%2-HHB-C 6.0%3-HHB-C 6.0%4-HHB-C 6.0%2-HHB(F)-C 5.0%3-HHB(F)-C 5.03%3-PyBB-F 7.0%4-PyBB-F 6.0%5-HBB-C 5.0%3-HB(F)EB(F)-C 5.0% Embodiment 9V2-HH-VFF(No.25) 10.0%2-BEB(F)-C 5.0%3-BEB(F)-C 7.0%4-BEB(F)-C 5.0%5-BEB(F)-C 7.0%103-HB(F)-C 6.0%3-HHEB(F)-F 5.0%4-HHEB(F)-F 5.0%5-HHEB(F)-F 10.0%2-HBEB(F)-C 5.0%3-HBEB(F)-C 5.0%4-HBEB(F)-C 5.0%5-HBEB(F)-C 5.0%3-HBTB-2 10.0%V2-HH-3 5.0%V2-HHB-1 5.0% Embodiment 10V2-HH-VFF(No.25) 15.0%1V2-BH-VFF(No.117) 5.0%V2-HB-C 3.0%4-BB-2 5.0%3-BB-C 5.0%5-BB-C 5.0%2-HB(F)-C 5.0%3-H2B-02 5.0%5-H2B-03 10.0%3-BEB-C 5.0%5-HEB-01 6.0%5-HEB-03 6.0%5-BBB-C 3.0%4-BPyB-C 3.0%4-BPyB-5 3.0%5-HB2B-4 4.0%5-HBB2B-3 4.0%V2-HH-101 3.0%1V2-HBB-3 5.0% Embodiment 11V2-HH-VFF(No.25) 5.0%V-HH-VFF(No.1) 5.0%5-H2B(F)-F 4.0%7-HB(F)-F 10.0%2-HHB(F)-F 12.0%3-HHB(F)-F 12.0%5-HHB(F)-F 12.0%2-H2HB(F)-F 12.0%3-H2HB(F)-F 6.0%5-H2HB(F)-F 12.0%2-HBB(F)-F 2.5%3-HBB(F)-F 2.5%5-HBB(F)-F 5.0% Embodiment 12V2-HH-VFF (No.25) 7.0%7-HB(F,F)-F 2.0%2-HHB(F)-F 10.0%3-HHB(F)-F 14.0%5-HHB(F)-F 14.0%2-H2HB(F)-F 4.0%3-H2HB(F)-F 2.0%5-H2HB(F)-F 4.0%3-HHB(F,F)-F 8.0%4-HHB(F,F)-F 4.0%3-H2HB(F,F)-F 6.0%4-H2HB(F,F)-F 5.0%5-H2HB(F,F)-F 5.0%3-HH2B(F,F)-F 8.0%5-HH2B(F,F)-F 7.0% Embodiment 131V2-HHH-VFF(No.41) 8.0%7-HB(F,F)-F 5.0%3-HBB(F,F)-F 5.0%5-HBB(F,F)-F 5.0%3-HHB(F,F)-F 7.0%5-HHB(F,F)-F 5.0%3-HH2B(F,F)-F 8.0%5-HH2B(F,F)-F 5.0%3-H2HB(F,F)-F 10.0%4-H2HB(F,F)-F 10.0%5-H2HB(F,F)-F 10.0%3-HHEB(F,F)-F 8.0%4-HHEB(F,F)-F 3.0%5-HHEB(F,F)-F 3.0%3-HBEB(F,F)-F 2.0%5-HBEB(F,F)-F 2.0%3-HHHB(F,F)-F 2.0%5-HH2BB(F,F)-F 2.0% Embodiment 141V2-BH-VFF(No.117) 5.0%V2-BH-VFF(No.27) 5.0%5-HB-F 2.0%7-HB(F)-F 3.0%2-HHB(F)-F 14.0%3-HHB(F)-F 14.0%5-HHB(F)-F 14.0%3-HB-02 5.0%3-HHB-F 4.0%3-HHB-1 6.0%3-HHB-3 6.0%2-HBB-F 6.0%3-HBB-F 5.0%3-HHEB-F 3.0%5-HHEB-F 3.0%3-HBEB-F 3.0%3-HHEBB-F 2.0% Embodiment 15V2-HH-VFF(No.25) 5.0%1V2-BHH-VFF(No.118) 3.0%7-HB(F,F)-F 7.0%3-HB-CL 5.0%7-HB-CL 5.0%2-BTB-01 10.0%2-HBB(F)-F 2.5%3-HBB(F)-F 2.5%5-HBB(F)-F 5.0%3-HBB(F,F)-F 7.0%5-HBB(F,F)-F 10.0%2-HHB-CL 5.0%3-HHB-CL 3.0%3-HB(F)TB-2 6.0%3-HB(F)TB-4 6.0%2-H2BTB-2 4.0%2-H2BTB-3 4.0%3-H2HB(F)-CL 4.0%5-H2HB(F)-CL 3.0%3-H2BB(F,F)-F 3.0% Embodiment 16V2-HH-VFF(No.25) 5.0%1V2-BH-VFF(No.117) 5.0%5-HB-F 10.0%6-HB-F 5.0%7-HB-F 5.0%2-HHB-OCF3 5.0%3-HHB-OCF3 5.0%5-HHB-OCF3 5.0%3-HH2B-OCF3 6.0%5-HH2B-OCF3 6.0%3-HB(F)B-3 4.0%5-HB(F)B-3 4.0%2-HBB(F)-F 10.0%3-HBB(F)-F 10.0%5-HBB(F)-F 15.0% Embodiment 17V2-HH-VFF(No.25) 6.0%V-HH-2VFF(No.116) 3.0%1V2-HH-VFF(No.37) 3.0%5-HB-F 3.0%6-HB-F 3.0%7-HB-F 3.0%3-HHB-OCF2H 7.0%5-HHB-OCF2H 7.0%3-HHB(F,F)-OCF2H 9.0%5-HHB(F,F)-OCF2H 9.0%2-HHB-OCF3 6.0%3-HHB-OCF3 6.0%4-HHB-OCF3 6.0%5-HHB-OCF3 6.0%3-HH2B(F)-F 7.0%5-HH2B(F)-F 7.0%3-HHEB(F)-F 4.0%6-HHEB(F)-F 5.0% Embodiment 18V2-HH-VFF(No.25) 15.0%3-HEB-04 23.4%4-HEB-02 17.6%5-HEB-01 17.6%3-HEB-02 14.7%5-HEB-02 11.7%TNI=67.7(℃)η=19.6(mPa·s) Embodiment 19V2-HHH-VFF(No.29) 15.0%3-HEB-04 23.4%4-HEB-02 17.6%5-HEB-01 17.6%3-HEB-02 14.7%5-HEB-02 11.7%TNI=B9.6(℃)η=25.7(mPa·s) Embodiment 20V2-HH-2VFF(No.115) 15.0%3-HEB-04 23.4%4-HEB-02 17.6%5-HEB-01 17.6%3-HEB-02 14.7%5-HEB-02 11.7%TNI=69.5(℃)η=20.5(mPa·s) Embodiment 21V2-HH-VFF(No.25) 10.0%V2-HH-2VFF(No.115) 8.0%1V2-BEB(F,F)-C 5.0%3-HB-C 25.0%1-BTB-3 5.0%3-HH-4 3.0%3-HHB-1 11.0%3-HHB-3 9.0%3-H2BTB-2 4.0%3-H2BTB-3 4.0%3-H2BTB-4 4.0%3-HB(F)TB-2 6.0%3-HB(F)TB-3 6.0%TNI=93.3(℃)η=13.9(mPa·s)Δn=0.147Vtn=2.08(V)0.8CM33 to 100,10.5μm。 Embodiment 22V2-HHH-VFF(No.29) 8.0%201-BEB(F)-C 5.0%301-BEB(F)-C 15.0%401-BEB(F)-C 13.0%501-BEB(F)-C 13.0%2-HHB(F)-C 15.0%3-HHB(F)-C 15.0%3-HB(F)TB- 24.0%3-HB(F)TB- 34.0%3-HB(F)TB- 44.0%3-HHB-01 14.0%TNI=94.5(℃)η=85.5(mPa·s)Δn=0.149Vtn=0.90(V) Embodiment 232-HH-VFF(No.25) 4.0%V2-HH-2VFF(No.115) 9.0%V2-HHH-VFF(No.29) 8.0%5-PyB-F 4.0%3-PyB (F)-F 4.0%2-BB-C 5.0%4-BB-C 4.0%5-BB-C 5.0%2-PyB-2 2.0%6-PyB-05 3.0%3-PyBB-F 6.0%4-PyBB-F 6.0%5-PyBB-F 6.0%3-HHB-1 6.0%2-H2BTB-2 4.0%2-H2BTB-3 4.0%2-H2BTB-4 5.0%3-H2BTB-2 5.0%3-H2BTB-3 5.0%3-H2BTB-4 5.0%TNI=99.4(℃)η=28.5(mPa·s)Δn=0.191Vtn=2.32(V) Embodiment 24V2-HH-VFF(No.25) 25.0%3-DB-C 10.0%4-DB-C 10.0%2-BEB-C 12.0%3-BEB-C 4.0%3-PyB(F)-F 6.0%5-HEB-02 4.0%5-HEB-5 5.0%4-HEB-5 5.0%10-BEB-2 4.0%3-HHB-1 6.0%3-HHEBB-C 3.0%3-HBEBB-C 3.0%5-HBEBB-C 3.0%TNI=67.3(℃)η=31.0(mPa·s)Δn=0.121Vtn=1.28(V) Embodiment 25V2-HH-2VFF(No.115) 4.0%3-HB-C 18.0%7-HB-C 3.0%101-HB-C 10.0%3-HB(F)-C 10.0%4-PyB-2 2.0%101-HH-3 7.0%2-BTB-01 7.0%3-HHB-1 7.0%3-HHB-F 4.0%3-HHB-01 4.0%3-HHB-3 8.0%3-H2BTB-2 3.0%3-H2BTB-3 3.0%2-PyBH-3 4.0%3-PyBH-3 3.0%3-PyBB-2 3.0%TNI=81.4(℃)η=16.9(mPa·s)Δn=0.137 Vtn=1.77(V) Embodiment 261V2-HH-VFF(No.37) 5.0%1V2-HH-2VFF(No.38) 8.0%201-BEB(F)-C 5.0%301-BEB(F)-C 12.0%501-BEB(F)-C 4.0%1V2-BEB(F,F)-C 10.0%3-HH-EMe 5.0%3-HB-02 10.0%7-HEB-F 2.0%3-HHEB-F 2.0%5-HHEB-F 2.0%3-HBEB-F 4.0%201-HBEB(F)-C 2.0%3-HB(F)EB-C 2.0%3-HBEB(F,F)-C 2.0%3-HHB-F 4.0%3-HHB-01 4.0%3-HHB-3 13.0%3-HEBEB-F 2.0%3-HEBEB-1 2.0% Embodiment 271V-HH-VFF(No.13) 5.0%1V-HH-2VFF(No.14) 5.0%V2-HHH-VFF(No.29) 8.0%201-BEB(F)-C 5.0%301-BEB(F)-C 12.0%501-BEB(F)-C 4.0%1V2-BEB(F,F)-C 15.0%3-HH-4 3.0%3-HHB-F 3.0%3-HHB-01 4.0%3-HBEB-F 4.0%3-HHEB-F 7.0%5-HHEB-F 7.0%3-H2BTB-2 4.0%3-H2BTB-3 4.0%3-H2BTB-4 4.0%3-HB(F)TB-2 5.0% Embodiment 28V2-HH-VFF(No.25) 20.0%V2-HHH-VFF(No.29) 4.0%2-BEB-C 12.0%3-BEB-C 4.0%4-BEB-C 6.0%3-HB-C 28.0%5-HEB-01 8.0%3-HEB-02 6.0%5-HEB-02 5.0%3-HHB-1 7.0%TNI=60.4(℃)η=18.2(mPa·s)Δn=0.112Vtn=1.31(V) Embodiment 291V2-HH-VFF(No.37) 5.0%1V-HH-VFF(No.13) 5.0%1V2-HH-2VFF(No.38) 5.0%1V-HH-2VFF(No.14) 5.0%2-BEB-C 10.0%5-BB-C 12.0%7-BB-C 7.0%1-BTB-3 7.0%10-BEB-5 12.0%2-HHB-1 4.0%3-HHB-F 4.0%3-HHB-1 7.0%3-HHB-01 4.0%3-HHB-3 13.0% Embodiment 30V2-HH-2VFF(No.115) 20.0%1V2-BEB(F,F)-C 6.0%3-HB-C 18.0%2-BTB-1 10.0%5-HH-VFF 10.0%1-BHH-VFF 8.0%1-BHH-2VFF 11.0%3-H2BTB-2 5.0%3-H2BTB-3 4.0%3-H2BTB-4 4.0%3-HHB-1 4.0%TNI=83.1(℃)η=11.7(mPa·s)Δn=0.132Vtn=2.10(V) Embodiment 31V2-HHH-VFF(No.29) 8.0%2-HB-C 5.0%3-HB-C 12.0%3-HB-02 15.0%2-BTB-1 3.0%3-HHB-F 4.0%3-HHB-01 5.0%3-HHB-3 14.0%3-HHEB-F 4.0%5-HHEB-F 4.0%2-HHB(F)-F 7.0%3-HHB(F)-F 7.0%5-HHB(F)-F 7.0%3-HHB(F,F)-F 5.0%TNI=103.0(℃)η=17.2(mPa·s)Δn=0.099Vtn=2.56(V) Embodiment 32V2-HH-VFF(No.25) 3.0%V2-HHH-VFF(No.29) 5.0%3-BEB(F)-C 8.0%3-HB-C 8.0%V-HB-C 8.0%1V-HB-C 8.0%3-HH-2V 14.0%3-HH-2V1 7.0%V2-HHB-1 15.0%3-HHEB-F 7.0%3-H2BTB-2 6.0%3-H2BTB-3 6.0%3-H2BTB-4 5.0%TNI=101.3(℃)η=15.2(mPa·s)Δn=0.132Vtn=2.25(V) Embodiment 33V2-HH-2VFF(No.115) 31.0%1V2-BEB(F.F)-C 12.0%3-HB-C 4.0%3-HB-02 5.5%3-HHB-1 3.5%1-BHH-VFF 20.0%3-HB(F)TB-2 4.0%3-HB(F)TB-3 4.0%3-HB(F)TB-4 4.0%3-H2BTB-2 4.0%3-H2BTB-3 4.0%3-H2BTB-4 4.0%TNI=100.4(℃)η=14.1(mPa·s)Δn=0.133Vtn=2.16(V)2.0CN to 100,10.6μm。 Embodiment 34V2-HH-VFF(No.2 5) 28.0%1V2-BEB(F,F)-C 12.0%3-HB-C 4.0%3-HB-02 5.5%3-HHB-1 8.5%1-BHH-VFF 20.0%3-HB(F)TB-2 5.0%3-HB(F)TB-3 5.0%3-H2BTB-2 4.0%3-H2BTB-3 4.0%3-H2BTB-4 4.0%TNI=100.5(℃)η=13.9(mPa·s)Δn=0.132Vtn=2.14(V)1.81CN to 100,12.3μm。 Embodiment 35
[0125] Symbolic representation of compounds The following examples relate to nematic liquid crystal compositions containing compounds of the present invention. Use Example 1 V2-HH-VFF (No.25) 30.0% V-BHH-VFF (No.7) 19.0% V-BBH-VFF (No.9) 15.0% 3-HB-C 5.0% 1V2-BEB ( F, F)-C 11.0% 3-HB-02 4.0% 3-HB(F)TB-2 4.0% 3-H2BTB-2 4.0% 3-H2BTB-3 4.0% 3-H2BTB-4 4.0% Application Examples 2V2-HH-VFF(No.25) 20.0%1V-BHH-VFF(No.19) 10.0%V-BBH-VFF(No.9) 6.0%3-HB-C 10.0%1V2-BEB(F,F )-C 12.0% 3-HB-02 5.0% 3-HHB-1 10.0% 3-HHB-3 10.0% 3-HB(F)TB-2 5.0% 3-H2BTB-2 4.0% 3-H2BTB-3 4.0 %3-H2BTB-4 4.0% Application Example 31V-BHH-VFF(No.19) 4.0%1V2-BHH-VFF(No.118) 10.0%V2-HB-C 10.0%1V2-HB-C 10.0%3 -HB-C 26.0% 5-HB-C 12.0% 3-HB(F)-C 8.0% 2-BEB-C 3.0% V2-HHB-1 8.0% 3-H2BTB-2 3.0% 3-H2BTB-3 3.0% 3-H2BTB-4 3.0% Application example 4V2-HH-VFF(No.25) 9.0% 1V2-BEB(F, F)-C 11.0% 201-BEB(F)- C 5.0% 301-BEB(F)-C 9.0% 3-HB(F)-C 15.0% 101-HH-3 3.0% 4-BTB-02 9.0% 2-HHB(F)-C 13.0% 3-HHB (F)-C 14.0% 3-H2BTB-2 4.0%...
Embodiment 1
[0135] Preparation of 1-(2,2-difluorovinyl)-trans-4-(trans-4-(3-butenyl)cyclohexyl)cyclohexane Formula (1) compound, number 25, wherein A 3 and A 4 Is anti-1,4-cyclohexylene; Z 3 is a covalent bond; Q 1 and Q 2 is H; Q 3 and Q 4 is F; l, n, p and q are 0; and m is 2)
[0136] first step
[0137] A mixture of 165.6 g (463.6 mmol) of methyltriphenylphosphonium bromide and 1.5 liters of THF was cooled to -50°C under nitrogen flow. 57.2 g (509.8 mmol) t-BuOK was added to the mixture and stirred for 1 hour. A solution of 100.0 g (356.6 mmol) trans-4-(trans-4-(2-formylethyl)cyclohexyl)cyclohexanecarboxylate in 1 liter of THF was added dropwise to the mixture while maintaining the temperature at -50 below ℃. After the dropwise addition, the reaction temperature was gradually raised to room temperature, and stirred for another 5 hours. The solvent was distilled off under reduced pressure, then 500 ml of water was added, and the mixture was extracted with 500 ml of toluene. ...
Embodiment 2
[0146] Preparation of 1-(4,4-difluoro-3-butenyl)-trans-4-(trans-4-(trans-4-vinylcyclohexyl)cyclohexyl)cyclohexane (compound (6), Formula (1), where A 2 , A 3 and A 4 Is anti-1,4-cyclohexylene; Z 2 and Z 3 is a covalent bond; Q 1 and Q 2 is H; Q 3 and Q 4 is F; l, m and p are 0; q is 1; n is 2)
[0147] first step
[0148]A mixture of 128.1 g (358.6 mmol) of methyltriphenylphosphonium bromide and 1.5 liters of THF was cooled to -50°C under a stream of nitrogen. 44.3 g (394.8 mmol) t-BuOK was added to the mixture and stirred for 1 hour. To the mixture was added dropwise a solution of 100.0 g (275.8 mmol) methyl 2-(trans-4-(trans-4-(trans-4-formylcyclohexyl)cyclohexyl)cyclohexyl)ethanecarboxylate in 1 liter of THF , while keeping the temperature below -50°C. After the dropwise addition, the reaction temperature was gradually raised to room temperature, and stirred for another 5 hours. The solvent was distilled off under reduced pressure, then 500 ml of water was adde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com