A kind of ruthenium complex and its preparation method and its application in detecting 5-formylcytosine
A formylcytosine and ruthenium complex technology, applied in the field of analysis and detection, can solve the problems of DNA sample degradation, limited resolution, low expression level, etc., and achieve the effect of simple conditions, wide response range, and fewer synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
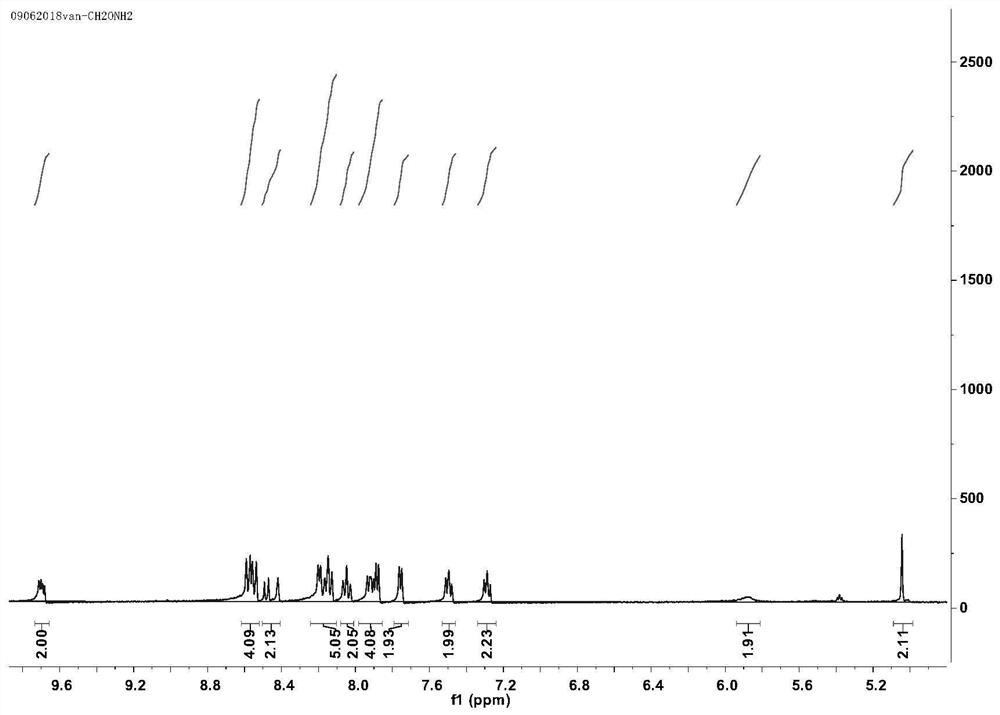
[0050] The synthesis of embodiment 1 ruthenium complex Ru-1C
[0051] (1) Weigh bis-(2,2'-bipyridine) (1,10-o-phenanthroline-5,6-dione) ruthenium (II) (1equ) hexafluorophosphate and dissolve it in acetonitrile (MeCN ), drop a few drops of acetic acid; dissolve 3,4-diaminobenzyl alcohol in acetonitrile and drop it into the previous system, cool after reflux for 2 hours, add excess saturated potassium hexafluorophosphate solution, centrifuge to obtain an orange-red precipitate, After drying in vacuum, purify by recrystallization to obtain bis-(2,2'-bipyridyl) hexafluorophosphate (bipyridyl[3,2-a:2',3'-c]phenazin-11-ylmethanol) Ruthenium(II);
[0052] (2) Di-(2,2'-bipyridyl) (dipyridyl[3,2-a:2',3'-c]phenazin-11-ylmethanol) obtained after the above purification ) ruthenium(II) was dissolved in acetonitrile, and excess phosphorus tribromide was added, after stirring at room temperature for 4 hours, an excess saturated potassium hexafluorophosphate solution was added, and an orang...
Embodiment 2
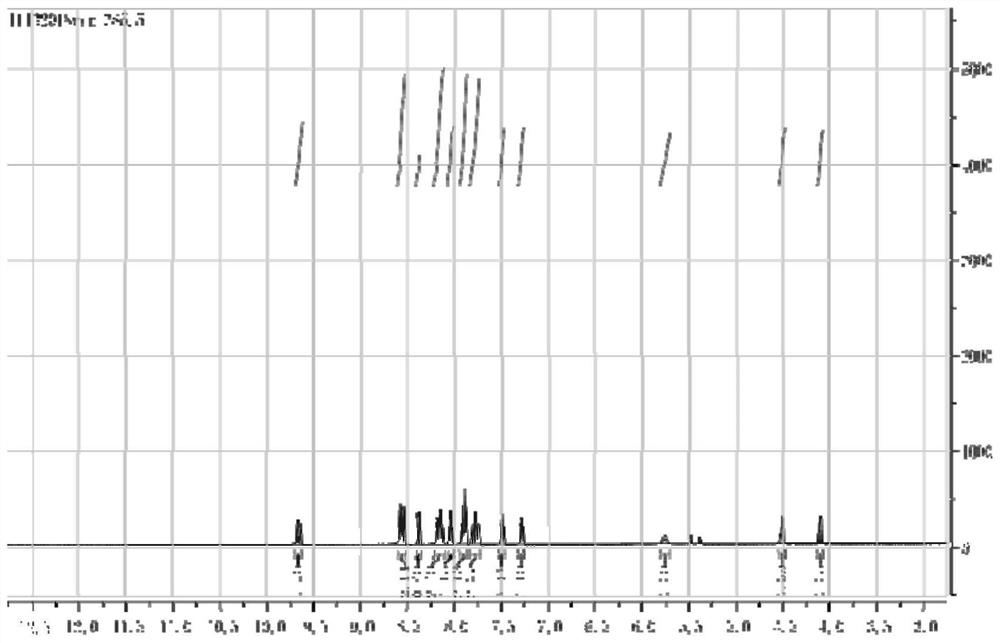
[0058] The synthesis of embodiment 2 ruthenium complexes Ru-2C
[0059] (1) Weigh bis-(2,2'-bipyridyl)(1,10-o-phenanthrene-5,6-dione)ruthenium(II)(1equ) hexafluorophosphate and dissolve it in DMF , drop a few drops of acetic acid; dissolve 2-(3,4-diaminophenoxy)ethyl bromide (1.5equ) in DMF first and then drop it into the previous system, reflux for 2 hours and cool, then add excess saturated six Potassium fluorophosphate solution, centrifuged to obtain an orange-red precipitate, vacuum dried, recrystallized and purified to obtain bis-(2,2'-bipyridyl)(11-(2-bromoethoxy)-bipyridyl[3, 2-a: 2',3'-c] phenazine) ruthenium (II);
[0060] (2) Then weigh bis-(2,2'-bipyridyl)(11-(2-bromoethoxy)-bipyridyl[3,2-a:2',3'-c]phenone hexafluorophosphate Azine) ruthenium (II), dissolved in DMF, adding excess sodium carbonate, then adding N-hydroxyphthalimide, stirring at room temperature overnight, adding excess saturated potassium hexafluorophosphate solution, and centrifuging to obtain an o...
Embodiment 3
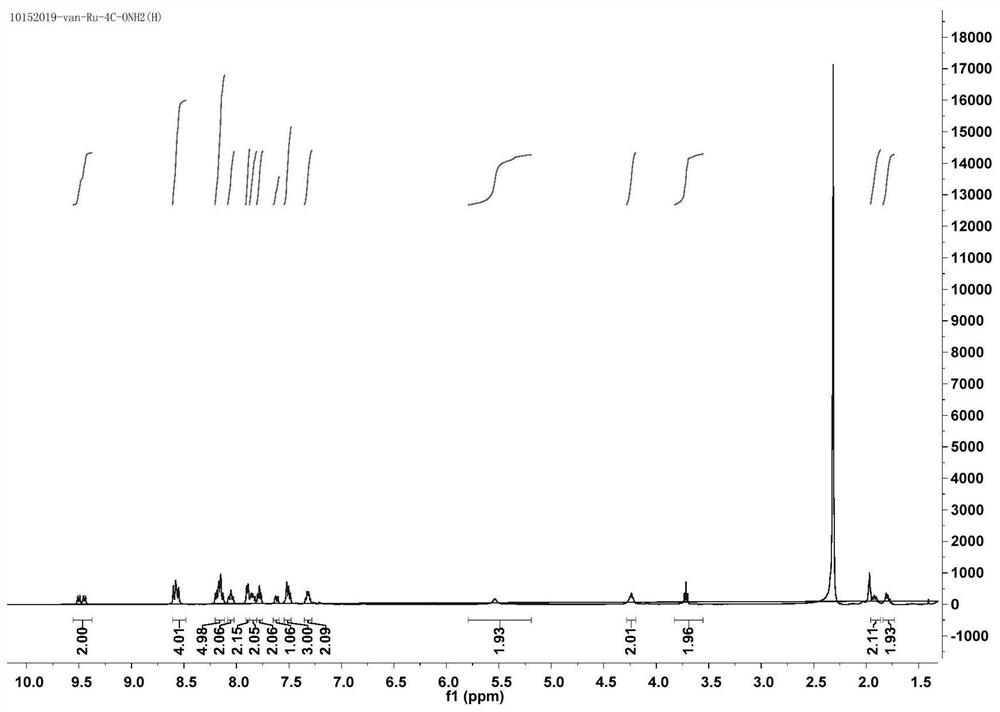
[0063] The synthesis of embodiment 3 ruthenium complexes Ru-4C
[0064](1) Weigh bis-(2,2'-bipyridyl)(1,10-o-phenanthrene-5,6-dione)ruthenium(II)(1equ) hexafluorophosphate and dissolve it in DMF , drop a few drops of acetic acid; dissolve 2-(3,4-diaminophenoxy)butyl bromide (1.5equ) in DMF first and drop it into the previous system, reflux for 2 hours and cool, add excess saturated six Potassium fluorophosphate solution, centrifuged to obtain an orange-red precipitate, after vacuum drying, recrystallized and purified to obtain bis-(2,2'-bipyridine)(11-(2-bromobutoxy)-bipyridine[3, 2-a: 2',3'-c] phenazine) ruthenium (II);
[0065] (2) Then weigh bis-(2,2'-bipyridyl)(11-(2-bromobutoxy)-bipyridyl[3,2-a:2',3'-c]phenone hexafluorophosphate Azine) ruthenium (II), dissolved in DMF, adding excess sodium carbonate, then adding N-hydroxyphthalimide, stirring at room temperature overnight, adding excess saturated potassium hexafluorophosphate solution, and centrifuging to obtain an ora...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com