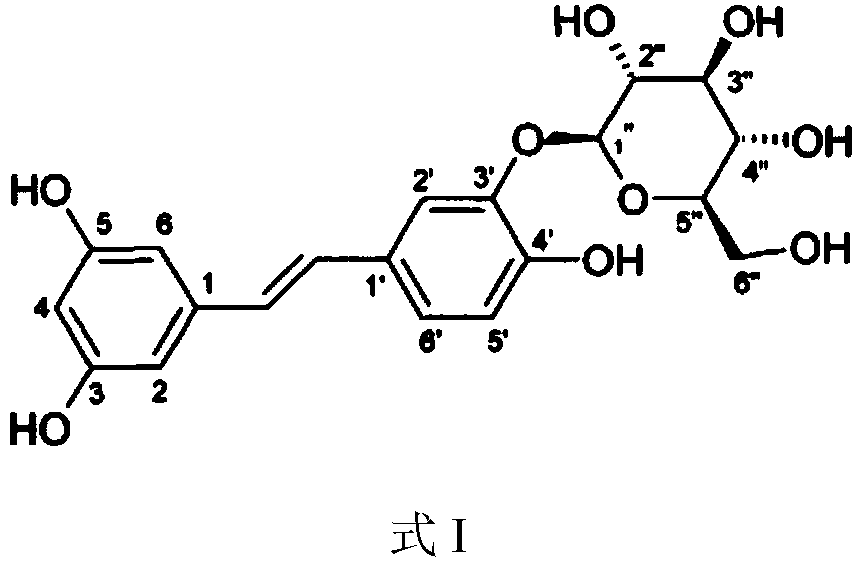
Application of Piceatannol 3'-O-glucoside in preparation of product for treating and/or preventing respiratory diseases
A technology for respiratory diseases and trizaperoside, which is applied in the field of application of trizaperoside in the preparation of products for the treatment and/or prevention of respiratory diseases, can solve problems such as unseen application reports, and achieve good market prospects and growth The effect of excretion amount and anti-inflammatory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1. Test material
[0033] 1.1 Animals
[0034] SPF grade ICR mice (weight: 20-24g) and guinea pigs (weight: 250-280g) are male and female, provided by the laboratory animal laboratory of Kunming Pharmaceutical Group Co., Ltd., production license: SCXK (Dian) K2014-0001 ;Mice are kept in IVC animal laboratory, guinea pigs are kept in normal-grade animal room, temperature is 20-26°C, humidity is 40%-70%, lighting is 12h: 12h alternating light and dark, illumination is 150-300lx, noise ≤60dB, used for experimental animals License: SYXK (Dian) K2014-0001, issuing unit: Kunming Science and Technology Bureau.
[0035] 1.2 Drugs and reagents
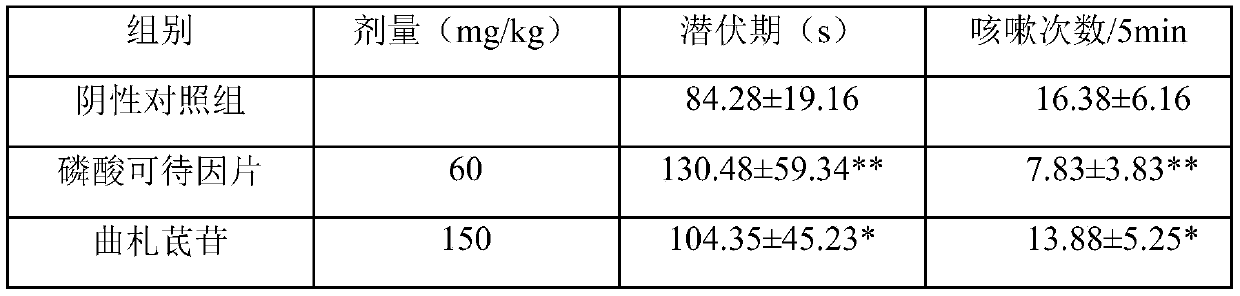
[0036] Tristilbene, batch number 20160301, the raw material was provided by the Pharmaceutical Research Institute of Kunming Pharmaceutical Group. Codeine Phosphate Tablets: Sinopharm Group Industry Co., Ltd., batch number 20180817. Ambroxol hydrochloride tablets: Sandoz (China) Pharmaceutical Co., Ltd., batch number FP264. Aminophy...
Embodiment 2
[0081] The tablet of this example consists of the following components: 50 g of tristilbene, 100 g of microcrystalline cellulose, 50 g of pregelatinized starch, 40 g of low-substituted hydroxypropylmethyl cellulose, and 1 g of micropowdered silica gel.
[0082] The above-mentioned raw materials were mixed, and trizaperine tablets were prepared according to conventional methods.
Embodiment 3
[0084] The capsule of this embodiment is composed of the following components: 25 g of tristilbene, 80 g of microcrystalline cellulose, 10 g of lactose, an appropriate amount of povidone K-30, and 2 g of magnesium stearate.
[0085] Mix the above-mentioned raw materials, and prepare tristilbene capsules according to conventional methods.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com