Hypoallergenic infant formula milk powder and preparation method thereof
An infant formula, hypoallergenic technology, applied in the direction of dairy products, whey, application, etc., can solve infant allergy and other problems, achieve the effect of promoting healthy development, protecting from infection and allergies, and reducing allergic symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-6
[0040] Embodiment 1-6 Hypoallergenic infant formula milk powder and preparation method thereof
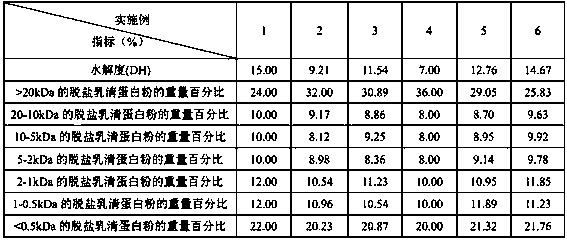
[0041] Examples 1-6 are hypoallergenic infant formula milk powders, and the raw materials for making their active ingredients are the same, the difference is that the amount of raw materials used in different examples is different, see Table 1 for details:
[0042] Table 1 Raw material ratio list
[0043]
[0044] In the above examples, the multivitamin contains the following components: vitamin A, vitamin D, vitamin E, vitamin K 1 , Vitamin B 2 , Vitamin B 6 , Vitamin B 12 , Niacinamide, folic acid, vitamin C, biotin, pantothenic acid;
[0045] Complex minerals contain the following components: sodium, potassium, copper, magnesium, iron, zinc, manganese, calcium, phosphorus, iodine, chlorine, selenium.
[0046] The following is the preparation method of the hypoallergenic infant formula milk powder in Example 1, which comprises the following steps carried out in sequence a...
Embodiment 7
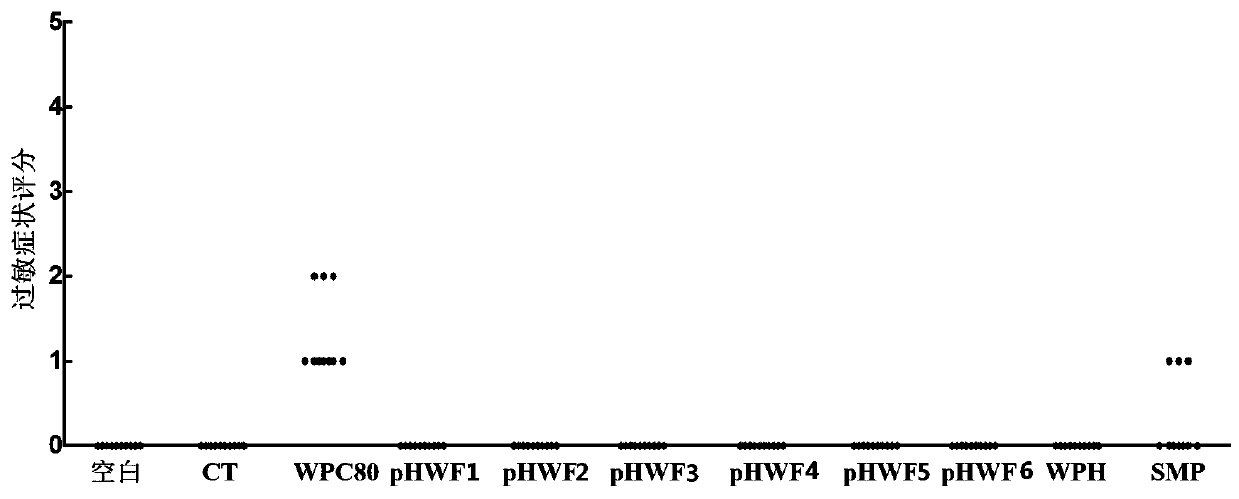
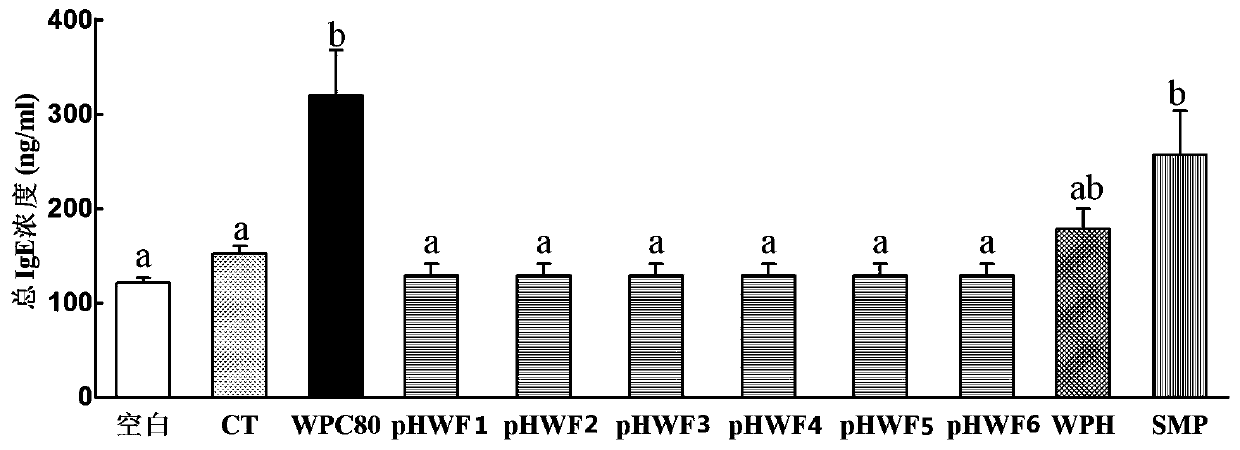
[0062] Example 7 Allergenicity Evaluation of Hypoallergenic Infant Formula Milk Powder
[0063] Select 110 4-week-old male BALB / c mice, and randomly divide them into 11 groups (n=11), which are blank group, CT group, 80% whey protein concentrate (WPC80) group, and prepared in Examples 1-6. Hypoallergenic infant formula (pHWF1-6) group, whey protein deep hydrolyzed powder (WPH) group and regular skimmed milk powder group (SMP). Among them, the WPC80 group was the positive control group, the blank group did not receive any treatment, and the CT group was only given Vibrio cholerae toxin (CT) 10 μg / cause, and the culture period was 6 weeks, once a week. The sensitizing dose of the experimental group was 20 mg / mouse for the first 5 gavages, supplemented with Vibrio cholerae toxin (CT) 10 μg / mouse to prevent the mice from developing immune tolerance, and the sensitizing dose for the last gavage was 50 mg Each group uses ultrapure water as the solvent, and the volume of intragastri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com