Alginic sodium diester sustained-release capsule and application thereof in treating cerebral ischemia-reperfusion injury
A technology of sodium alginate and sustained-release pellets, which is applied in the treatment of cerebral ischemia-reperfusion injury. In the field of sodium alginate sustained-release capsules, it can solve the problem of inability to maintain stable and effective blood drug concentration and biological half-life Short, short action time and other issues, to achieve the effect of inhibiting the formation of thrombus, lowering blood lipids, inhibiting the occurrence and development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1. Investigation of enteric-coated materials
[0046] The enteric-coated material of the present invention is selected from HPMCAS, HPMCP, Eudragit S100, Eudragit RL100, and Eudragit RS100 as the enteric-coated material, and the influence of different enteric-coated materials on the extrusion process and the release of the drug in vitro is investigated. Among them, HPMCAS has the smallest torque during extrusion, and the color of the extrudate is the most similar to that of the API, and there is no polymerization phenomenon in the pH1.0 medium, indicating that the enteric-coated material has a good wrapping effect on the main drug, preventing ring-opening polymerization of the main drug. Therefore, HPMCAS is preferred as the enteric carrier material.
[0047] 2. Proportion inspection
[0048] The ratio of the main drug to the enteric material usually affects the feasibility of extrusion, the dissolution and stability of the drug. In this experiment, the mass ratio of t...
Embodiment 2
[0052] Investigate the dosage of main and auxiliary materials, and use the orthogonal experimental design method to screen the dosage of main and auxiliary materials:
[0053] The main drug is sodium alginate: 10-20 parts;
[0054] Enteric-coated material: 10-20 parts, one or more selected from HPMCAS, HPMCP, Eudragit S100, Eudragit RL100, and Eudragit RS100; HPMCAS is preferred as the enteric-coated material.
[0055] Diluent: 20-30 parts, one or more selected from starch, powdered sugar, lactose, β-cyclodextrin, and microcrystalline cellulose; microcrystalline cellulose is not absorbed in the body, and its compressibility It has good fluidity and has a certain capillary action. When it meets water, it can make water quickly enter the core of the ball and destroy the combination between the particles, which is helpful for the rapid disintegration of the drug. Therefore microcrystalline cellulose is preferably used as diluent.
[0056] Binder: 2 to 10 parts, one or more sele...
Embodiment 3
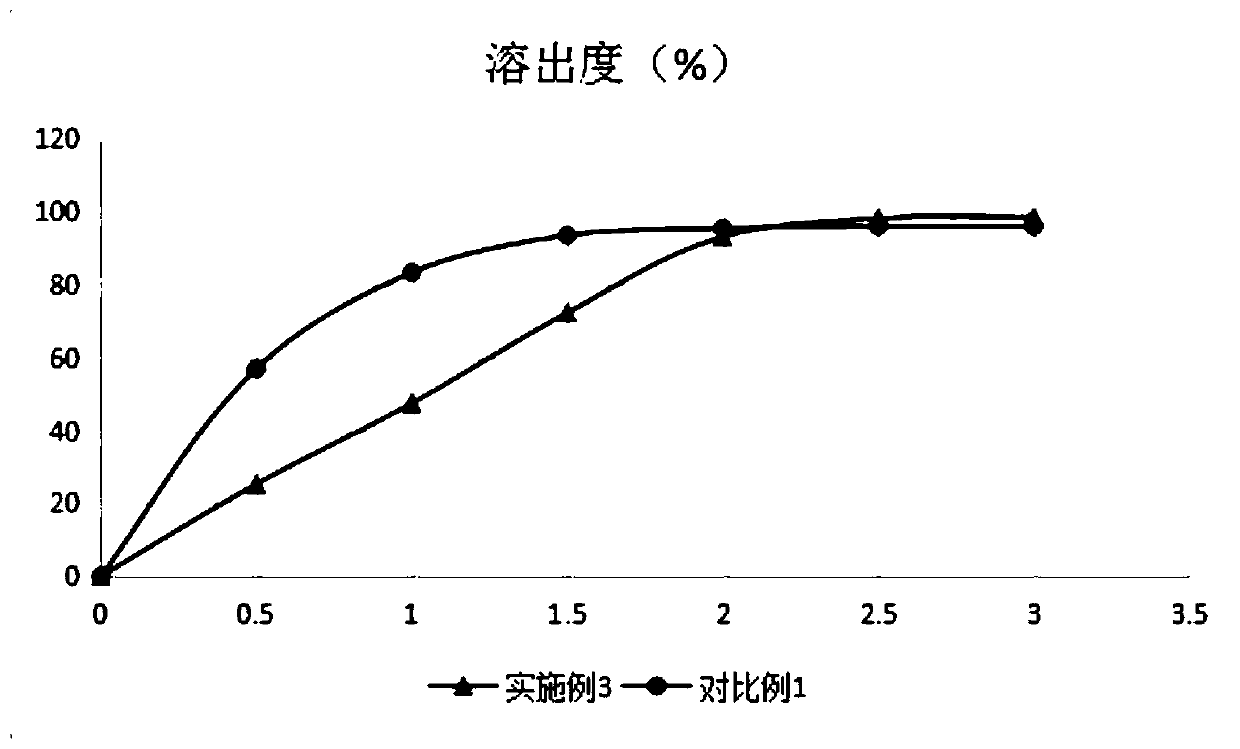
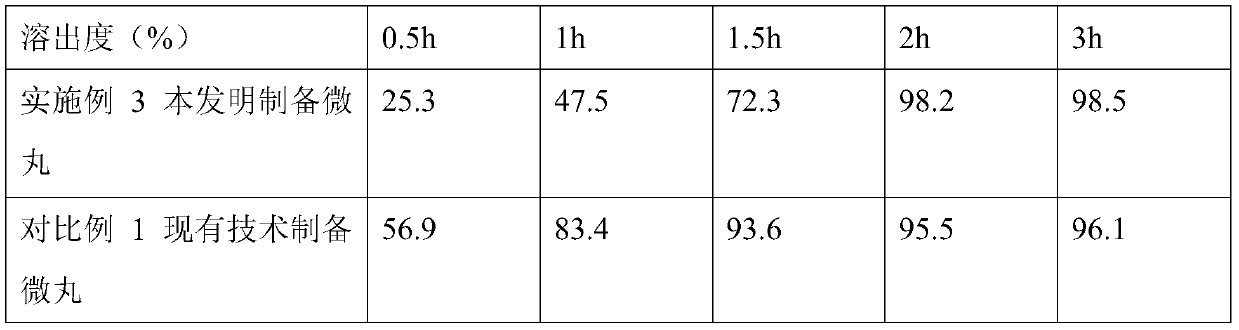
[0066] Embodiment 3 The preparation of sustained-release capsules of the present invention, based on 500 calculations, the specification is 30mg,
[0067] The prescription for medicated pill cores is as follows:
[0068] Sodium alginate: 15g,
[0069] HPMCAS: 15g,
[0070] Microcrystalline cellulose: 25g,
[0071] Polyvinylpyrrolidone: 8g, selected from polyvinylpyrrolidone,
[0072] Di-tert-butylhydroxytoluene: 2g, selected from di-tert-butylhydroxytoluene,
[0073] Magnesium stearate: 2g,
[0074] Enteric coating liquid prescription is as follows:
[0075] HPMCP: 10g,
[0076] castor oil 2g,
[0077] Sodium Lauryl Sulfate: 2g,
[0078] Water: Appropriate amount,
[0079] Coating weight gain 10%;
[0080] The preparation method of sodium alginate sustained release capsule comprises the following steps:
[0081] Step 1, preparation of skeleton-type drug-containing pellets: use sodium alginate as the raw material, enteric-coated materials and pharmaceutically accepta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com