A heterologous antibody against giant panda canine distemper virus and its preparation method
A canine distemper virus, giant panda technology, applied in the field of antibody and antibody preparation, can solve the problems of economic loss, allergic reaction to heterologous protein rejection, expression level, production scale and purification ability lag, etc. High and safe effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
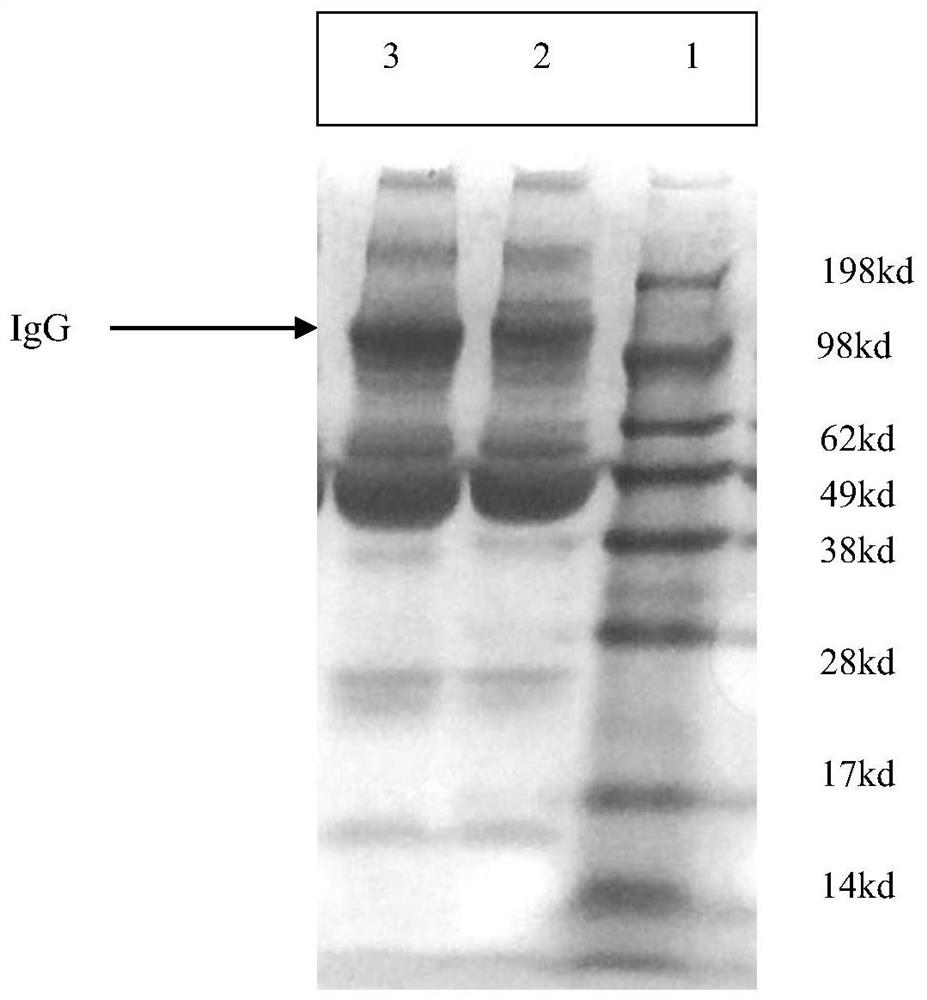
Image
Examples
Embodiment 1
[0026] Embodiment 1: the preparation of giant panda canine distemper inactivated vaccine
[0027] (1) Preparation and inspection of seed batches of giant panda canine distemper virus strain
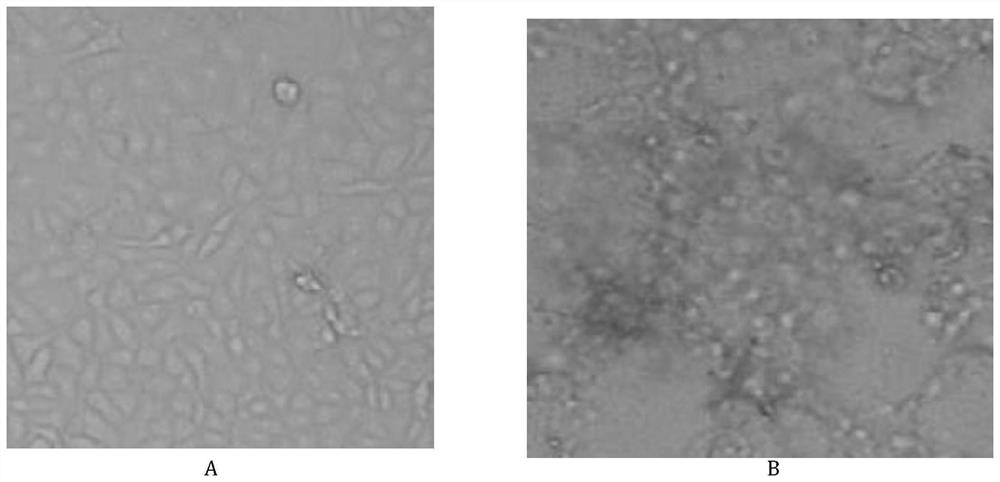
[0028] The giant panda canine distemper virus strain was inoculated on a monolayer of Vero cells at 0.5%, and the virus was cultured at 37°C, and the virus supernatant was harvested when the cytopathy reached 80% to 90%, and the passage was 1 generation , and so on. Obvious cytopathic changes appeared at passage 5 (see figure 1 ). According to the above method, the giant panda canine distemper virus strain was continuously passaged in Vero cells up to 30 generations. Carry out virus content determination (see Table 1) to each generation, carry out sterility test, mycoplasma test, exogenous virus test, virulence test, specificity test and immunogenicity test to 1, 10, 15, 20, 24 generations , the test results are all qualified. It is finally determined that the highest generation of t...
Embodiment 2
[0071] Example 2: Preparation and collection of horse anti-, cat anti-, dog anti-canine distemper virus hyperimmune serum
[0072] Quarantine was carried out in accordance with relevant standards, and 4 healthy antibody-negative horses, 5 healthy antibody-negative 4-6-month-old kittens and 5 healthy antibody-negative 1-year-old Beagle dogs were selected, and they were subcutaneously passed through the back of the body respectively. The method of split-point injection was used for primary immunization and multiple booster immunizations. A total of 4 immunizations were carried out, with an interval of 14 days between each time. 0.3mg, 0.4mg, 0.5mg / cat, 0.2mg, 1.0mg, 1.5mg, 2mg, 3mg / dog. Before each immunization, venous blood was collected, the serum was separated, and the neutralizing antibody titer was tested. When the neutralizing antibody titer was above 1:10,000, the blood was collected in line with high immune serum, the serum was aseptically separated, and stored at -20°...
Embodiment 3
[0086] Example 3: Safety test of purified heterologous antibody against giant panda canine distemper virus
[0087] 1. Safety test of a single dose of immunization in dogs
[0088] Divide 15 Beagle dogs into 3 groups, 5 in each group, inject purified horse-derived antibody, cat-derived antibody and canine-derived antibody intramuscularly, 1 head / dog each time, and set another 15 Beagle dogs for three The control group was used as the control group without immunization, and was observed for 14 days. The general symptoms, body weight, body temperature and injection site of the dogs were observed 1 day before immunization and within 14 days of immunization. The results showed that after the Beagle dogs were immunized with a single dose, there were no abnormal clinical manifestations locally and systemically after the injection of the horse-derived and canine-derived antibodies, normal weight gain, no abnormal body temperature, and no significant difference from the control group...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com