Acinetobacter baumannii omp22 recombinant multi-antigen epitope polypeptide and its application
A technology of Acinetobacter baumannii and epitope polypeptide, applied in A.bOmp22 recombinant multi-antigen epitope polypeptide and application field, can solve problems such as toxicity and harm, and achieve the effect of avoiding toxic effect, reducing infection and epidemic
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
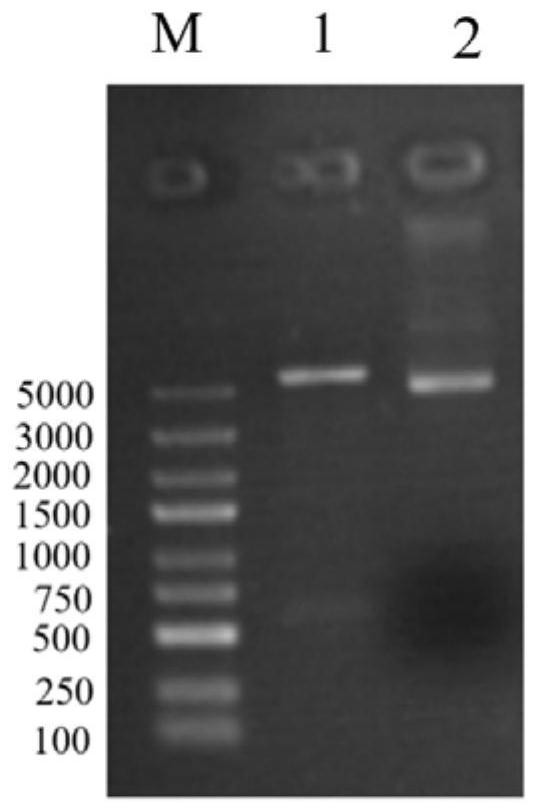
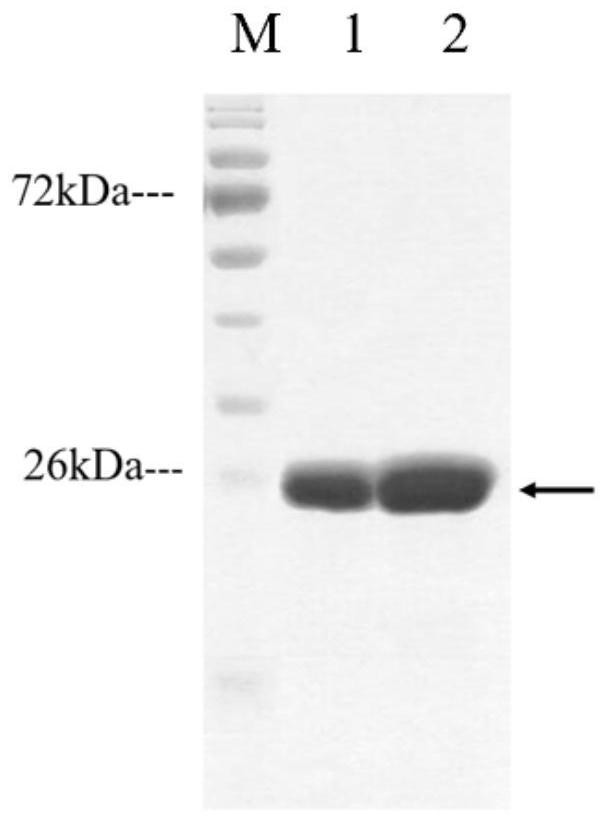
[0045] A.b Construction and screening of polypeptide sequences of B cell antigen epitope and T cell antigen epitope of Omp22 protein, and chemical synthesis of recombinant multi-antigen epitope polypeptide rOmp22. Specific steps are as follows:
[0046] The amino acid sequences of 4 B-cell epitopes and 4 T-cell epitopes of Acinetobacter baumannii Omp22 protein are:
[0047] A.b Omp22 175-182: GKGVPSSR;
[0048] A.b Omp22 158-172: NIPLSQARAQSVKNY;
[0049] A.b Omp22 125-135: YATLDKVAQTL;
[0050] A.b Omp22 102-108: SVQLIMP;
[0051] A.b Omp22 112-122: TFDTNKSNIKP;
[0052] A.b Omp22 153-164: GNDSINIPLSQ;
[0053] A.b Omp22 178-188: VPSSRIDAQGY;
[0054] A.b Omp22 204-215: EQNRRVEISIY.
[0055] Entrust Nanjing KingScript Biotechnology Co., Ltd. to chemically synthesize, with a purity of more than 85%, and couple BSA molecules to the C-terminus of each epitope short peptide;
[0056] A. Construct the recombinant plasmid pET28a-omp22, transform the recomb...
Embodiment 2
[0070] For the detection of the immune effect of the recombinant multi-antigen epitope polypeptide rOmp22, the specific implementation steps are as follows:
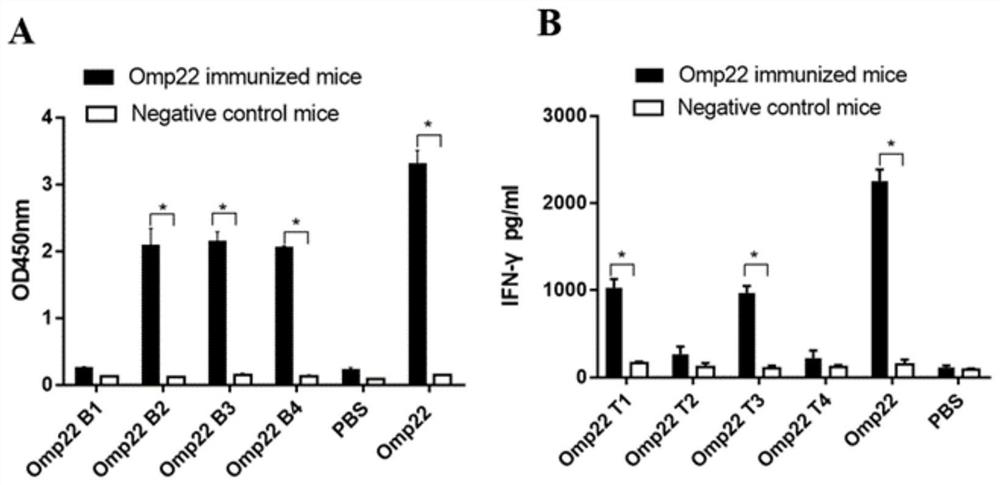
[0071] Female BALB / c mice at 6-8 weeks were randomly divided into 4 groups, 6 in each group, respectively high-dose rOmp22 group (40ug / mouse), low-dose rOmp22 group (10ug / mouse), and Fu Adjuvant 1:1 was mixed and fully emulsified. A normal saline + adjuvant group and a normal saline control group were set up, with a total volume of 200ul. They were injected subcutaneously for immunization. Complete Freund's adjuvant was used for the first immunization, and Freund's non-toxic adjuvant was used for the last two immunizations. Complete adjuvant, once every two weeks, a total of three immunizations. One week after the last immunization, the mouse serum was collected and the spleen was isolated.
[0072] The ELISA method is used to detect the expression level of rOmp22-specific IgG in mouse serum. The steps are as follows: 2...
Embodiment 3
[0075] Recombinant multi-antigen epitope polypeptide rOmp22 pair A.b The resulting mouse pneumonia has an immune protective effect, and the specific implementation steps are as follows:
[0076] 6-8 weeks female BALB / c mice were randomly divided into 4 groups (10 mice in each group), respectively high-dose rOmp22 group (40ug / mouse), low-dose rOmp22 group (10ug / mouse), and Freund's adjuvant was mixed and fully emulsified at 1:1, and a normal saline + adjuvant group and a normal saline control group were set up, with a total volume of 200ul, and were injected subcutaneously for immunization. Freund's complete adjuvant was used for the first immunization, and Freund's for the last two immunizations. Incomplete adjuvant, once every two weeks, a total of three immunizations.
[0077] 2 weeks after the last immunization, 50ul half-lethal dose of A.b Standard strain ATCC 19606 (1×10 8 CFU, containing 5% porcine mucin), to establish a mouse model of acute pneumonia.
[0078] 24 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com