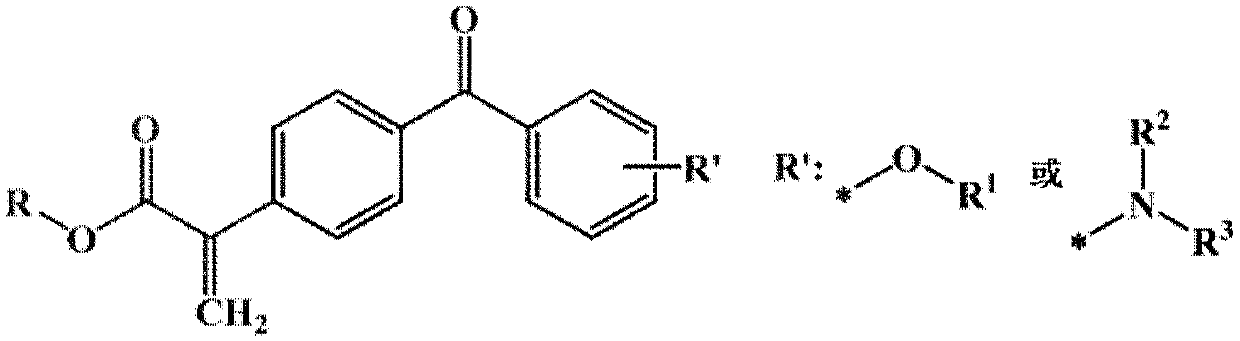
High-efficiency polymerizable photoinitiator for photoresistance
A photoinitiator and polymerization type technology, applied in the field of photoinitiator for polymerizable color photoresist and its preparation, can solve the problems of easy yellowing, low solubility, large amount of photoinitiator, etc. The effects of migration, increased crosslink density, and increased initiation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
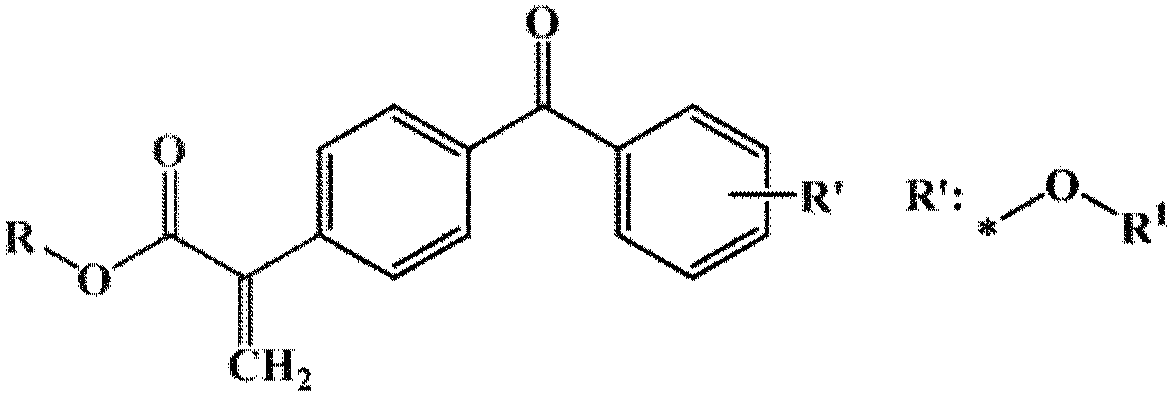
[0046] Example 1. 4-methoxy,4'-(2-methacrylate)benzophenone E-1 Synthesis
[0047]
[0048] Add 48g of methanol (1.5mol) into a 250mL three-necked flask and stir, raise the temperature to reflux, slowly add 42g of diketene (0.5mol), continue to react at reflux temperature for 5h, and distill under reduced pressure to obtain 89.4g (0.77mol) of methyl acetoacetate .
[0049] Add 200mL THF and 15.6g stirred and dispersed NaH (60%, dispersed in mineral oil, 0.39mol) to a 500mL three-necked flask protected by argon, and 34.8g methyl acetoacetate (0.3mol) is added dropwise at room temperature in the flask and stir well. When the liquid in the flask became clear, 46.8 g of iodomethane (0.33 mmol) was added dropwise, the mixture was stirred at 40° C. for 10 h, the reaction solution was quenched with saturated aqueous ammonium chloride and extracted 3 times with ethyl acetate. The combined organic layers were dried with anhydrous sodium sulfate and spin-dried to dry the solvent,...
Embodiment 2
[0066] Example 2. 4-tert-butoxy,4'-(2-ethylacrylate)benzophenone E-2 Synthesis
[0067]
[0068] The synthesis process of E-2 refers to the synthesis of E-1, the difference is that the methanol used in A-1 is replaced by ethanol, and the anisole used in C-1 is replaced by tert-butylphenyl ether.
[0069] Wherein: tert-butylphenyl ether: purity 98%, purchased from Alfa Aesar.
[0070] NMR data of E-2: 1 H NMR (300Hz) in DMSO: δ1.07ppm (t, 3H), 1.42ppm (s, 9H), 4.00ppm (q, 2H), 6.20, 6.39ppm (d, J = 56.7, 2H), 7.03-7.73 ppm(m,8H).
Embodiment 3
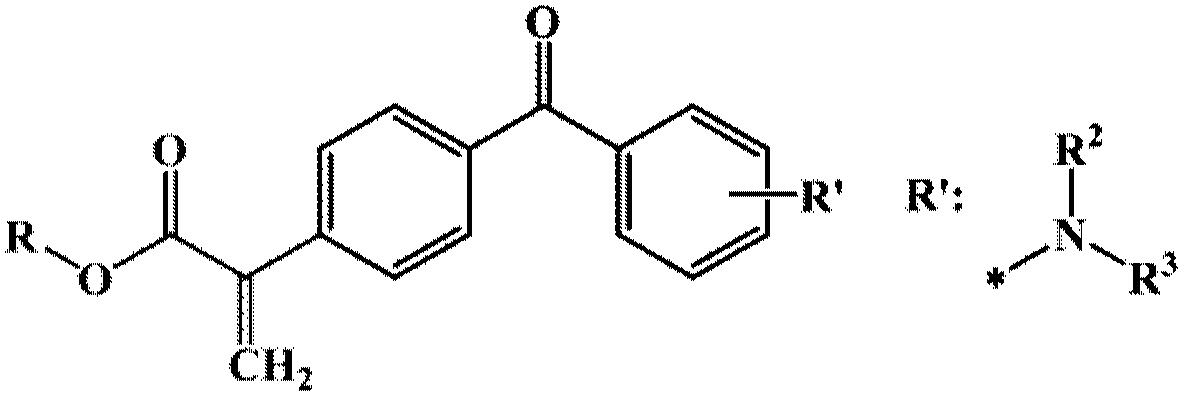
[0071] Example 3. 4-(N,N-Dimethylamino),4’-(2-Acrylic acid methyl) benzophenone E-3 Synthesis
[0072]
[0073]Under nitrogen protection, add 7.2g of magnesium chips (0.3mol) and a grain of iodine to a 500mL three-necked flask, slowly introduce 100mL of THF solution and 20g of 4-bromo-N,N-dimethylaniline (0.1mol) into the flask . Control the Grignard reaction speed, and after the dripping is completed, heat in a water bath until THF refluxes. After 4 hours, the heating was removed, and after the temperature of the liquid in the flask dropped to room temperature, the solution in the flask was slowly introduced into a 1000 mL three-necked flask through a thin guide needle. Fully dissolve 20 g of 4-bromooxynil (1.1 mol) in 100 mL of THF solution, and slowly drop it into the three-neck flask. After the dripping was completed, the solution in the flask was heated to THF reflux, and the water bath was removed after the reaction was completed. After the solution in the flask d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com