Method for preparing diazoxide
A diazoxide and reaction technology, applied in the field of compound preparation, can solve the problems of violent reaction, easy moisture absorption, long steps, etc., and achieve the effect of mild reaction conditions, short reaction steps and stable reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The method for preparing diazoxide disclosed by the present invention is as follows:
[0033] Mix 2-amino-5-chlorobenzenesulfonamide, imidazolium salt and amide solvent and react with heating to obtain diazoxide. The preparation method of further 2-amino-5-chlorobenzenesulfonamide is as follows, anthranilamide and N - Chlorosuccinimide is reacted in a solvent to give 2-amino-5-chlorobenzenesulfonamide.
[0034] or
[0035] Mix anthranilamide, imidazolium salt and amide solvent and heat to react to obtain compound IV; then compound IV and N - Chlorosuccinimide is reacted in a chlorine solvent to give diazoxide.
[0036]
[0037] The column chromatography purification of all embodiments adopts ethyl acetate / petroleum ether with a volume ratio of 1:1 as eluent, R f is 0.3.
Embodiment 1
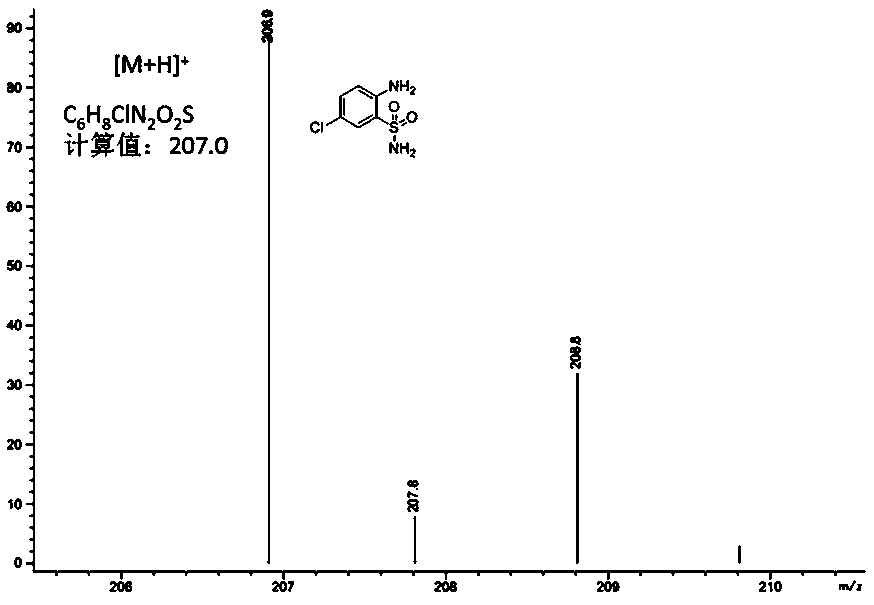
[0039] Anthranilamide (1.72g) was mixed with NCS (1.32g, 1.0 equivalent), dissolved in dichloromethane, stirred at reflux temperature for 5 hours, and then purified by column chromatography to prepare compound II 2-amino -5-chlorobenzenesulfonamide, 1.75g, the yield of this step is 85%, and the purity is greater than 99%. Compound Mass Spectrum: Calculated [M+H] + C 6 h 8 ClN 2 o 2 S, 206.99; experimental value: 206.83.
[0040] Anthranilamide (1.72g) was mixed with NCS (1.32g, 1.0 equivalent), dissolved in chloroform, stirred at reflux temperature for 3 hours, and then purified by column chromatography to prepare compound II2-amino-5- Chlorobenzenesulfonamide 1.96g, the yield of this step is 95%, the purity is greater than 99%, the experimental value of mass spectrum: 206.9, see the mass spectrum figure 1 .
Embodiment 2
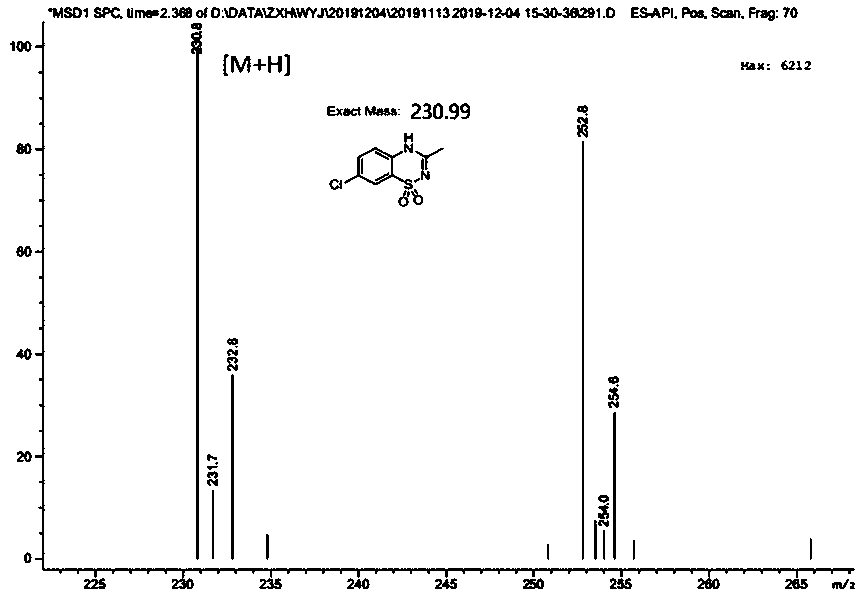
[0042] Dissolve 2-amino-5-chlorobenzenesulfonamide (2.05g) in 8.0ml N , N -In dimethylacetamide, add 0.14g (10mol%) imidazole hydrochloride again, reaction temperature 120 o C, after stirring for 48 hours, the reaction solution was distilled, and the excess N , N -Dimethylacetamide was recovered by distillation, and the residue was purified by column chromatography to obtain 2.07 g of the product diazoxide (compound III), with a yield of 90%, a purity greater than 99%, and an experimental value of mass spectrometry: 230.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com