Nano-antibody GN1 specifically combined with GPC3 protein and preparation method and application thereof
A nanobody, GN1 technology, applied in the field of nanobody GN1 and its preparation, can solve the problems of insufficient stability, hindering the sensitivity of the antibody, and it is difficult for the antibody to achieve affinity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: Preparation of Nanobody GN1
[0066] The preparation process of Nanobody GN1 comprises the following steps:
[0067] (1) Immune alpaca:
[0068] Take 1 mg of GPC3 protein expressed by eukaryotic HEK293 cells and emulsify with complete Freund's adjuvant, totaling 2 mL, and inject a healthy adult alpaca for the first time by subcutaneous multi-point injection; on the 15th day, 0.5 mg of GPC3 protein Emulsified with Freund's complete adjuvant, a total of 2mL, subcutaneous multi-point injection for the second immunization; then use 0.5mg GPC3 protein emulsified with Freund's incomplete adjuvant to obtain a total of 2mL of immunization emulsified injection every 7 days for next immunization Immunity once. A total of 6 immunizations were performed, and blood was collected on the 7th day after each immunization to detect the titer. After testing the serum titer, 100 mL of peripheral blood was collected. The inventors found that the protein expressed by eukaryoti...
Embodiment 2
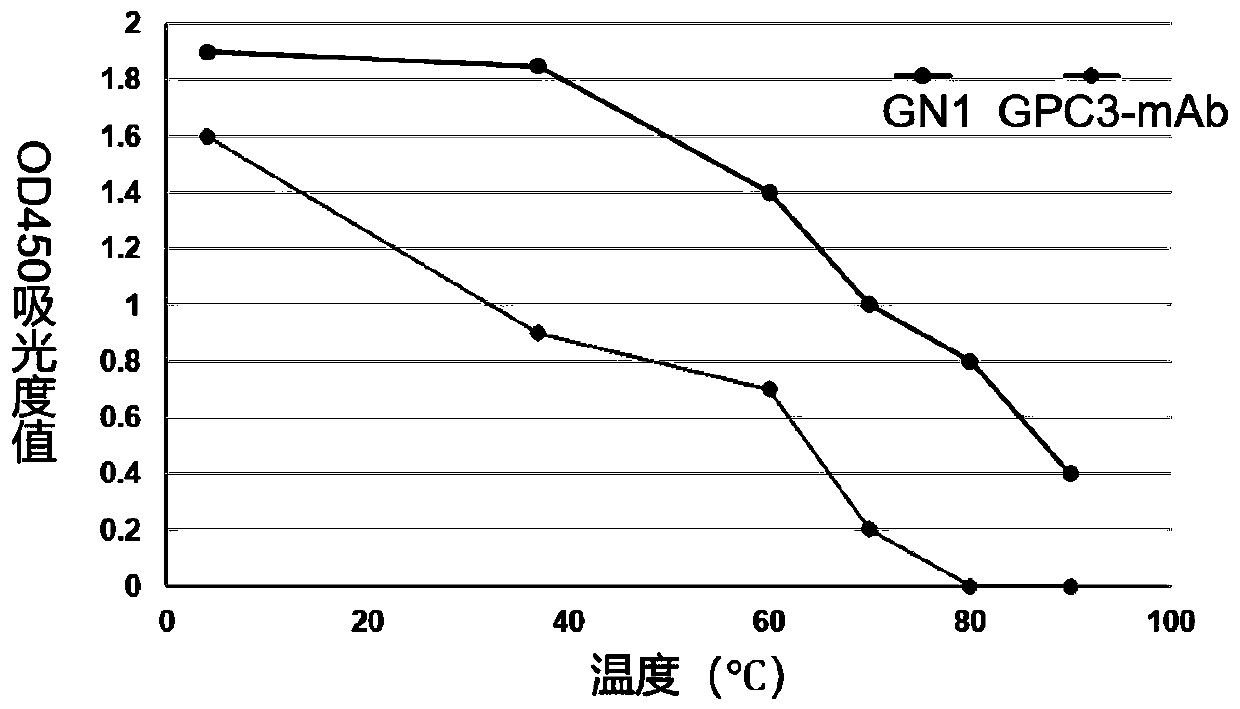
[0092] Embodiment 2: Nanobody GN1 thermal stability experiment:
[0093] (1) Coating GPC3 protein, 1 μg / mL GPC3 protein, 100 μL per well was added to the microtiter plate, and coated overnight at 4°C.
[0094] (2) After washing the plate three times with PBST, add 300 μL of 5% skimmed milk to each well, and block at 37° C. for 1 hour.
[0095] (3) Add Nanobody GN1 of the present invention and commercialized GPC3 monoclonal antibody (GPC3-mAb, Invitrogen) to each well for 2 hours at 4°C, 37°C, 60°C, 70°C, 80°C, and 90°C. . 100 μL per well, after incubating at room temperature for 1 hour, wash the plate 3 times with PBST.
[0096] (4) In the GN1 group, because the GN1 antibody has a HA tag, add HRP enzyme-labeled HA-mAb (SANTA CRUZ company) to each well, incubate at room temperature for 40 minutes, wash the plate three times with PBST, add TMB for 10 minutes, and use 2M After sulfuric acid terminates the reaction, the microplate reader detects the ultraviolet absorbance (OD45...
Embodiment 3
[0098] Example 3: Establishment of a sandwich ELISA method for detecting serum GPC3 protein with GN1 nanobody-based GN1-luciferase fusion protein:
[0099] (1) Amplify the GN1 gene. Using the plasmid obtained in step (4) of Example 1 as a template, the GN1 nucleotide sequence was amplified by PCR; the PCR product was digested with Nco I and Sfi I, and the PCR product purification kit was used to purify and recover the digested product.
[0100] (2) Construction of fusion gene GN1-Luc. The synthetic fluorescent reporter gene is Nano-luciferase gene (Nano-luciferase, referred to as Luc) (the amino acid sequence of the fluorescent protein reporter gene luciferase is shown in SEQ ID NO.14): subcloned into the NotI and SalI sites of the vector pET22b Between, Nco I and Sfi I double digest the vector pET22b that contains fluorescent reporter gene nano-luciferase, cut the gel and reclaim the pET22b vector backbone sequence that contains luciferase gene; The vector backbone sequence ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com