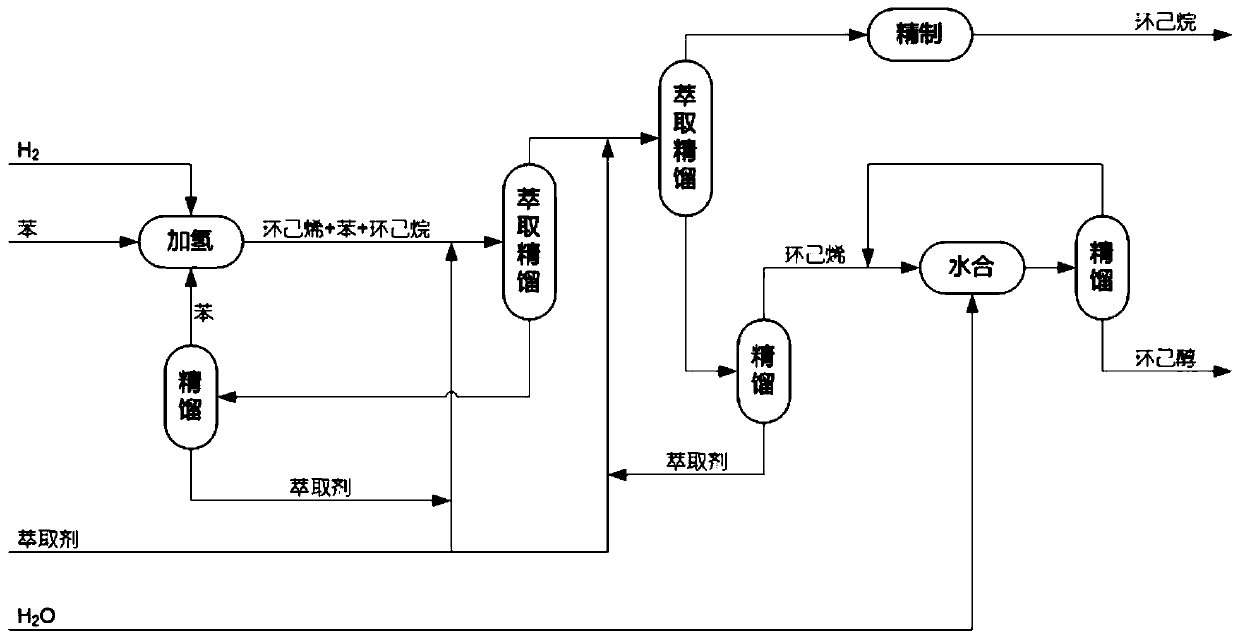
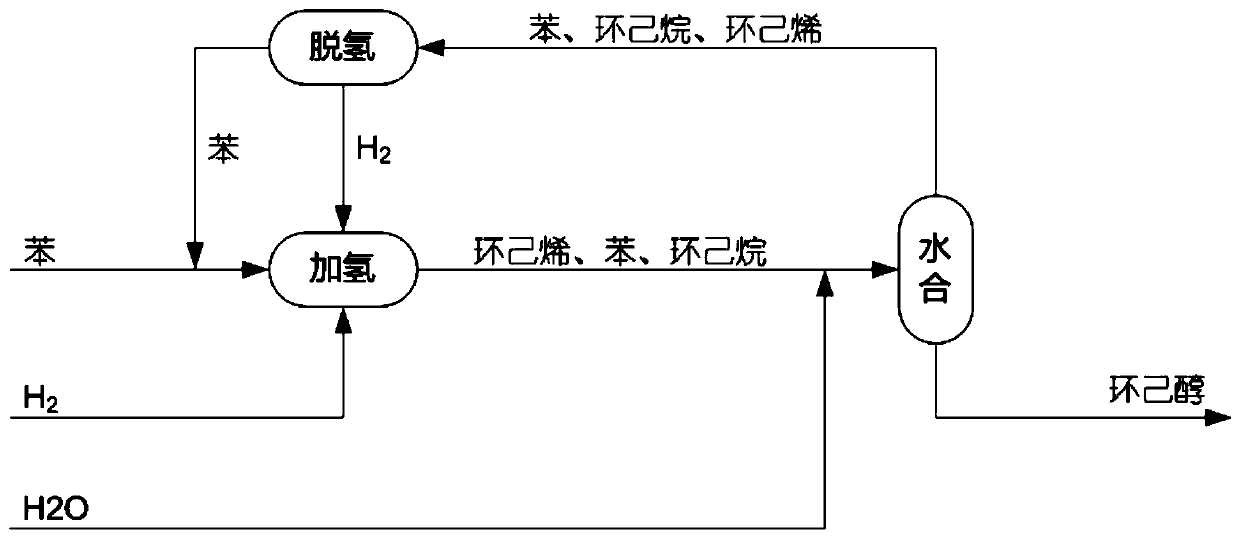
Preparation method of cyclohexanol through dehydrogenation with coupling of cyclohexane mixture
A cyclohexane and mixture technology, applied in the field of cyclohexanol preparation, can solve the problems of high energy consumption, large equipment investment, complex process flow, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
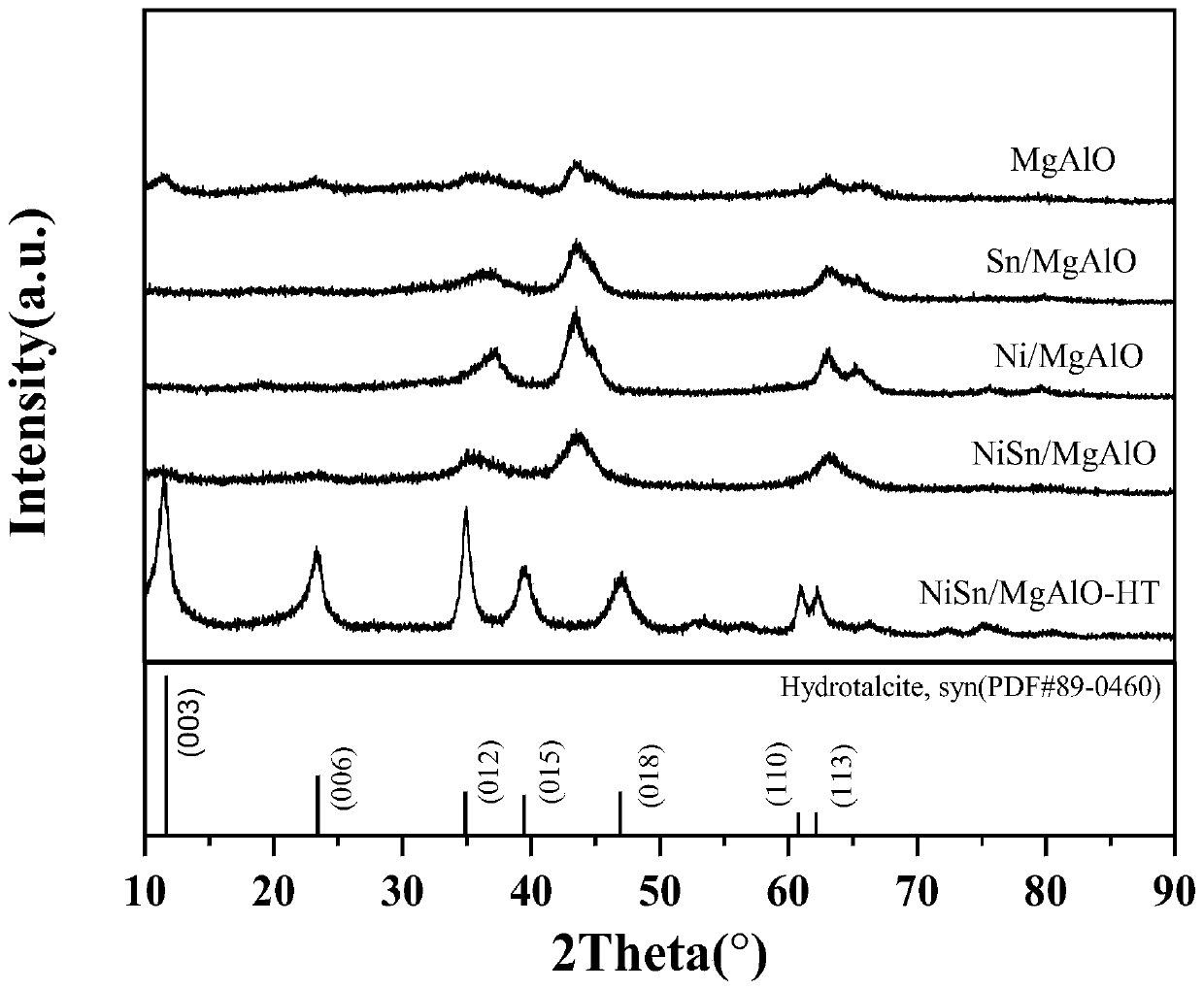
[0062] The preparation process of the dehydrogenation catalyst is as follows: use a certain amount of deionized water to dissolve the magnesium nitrate and aluminum nitrate metal salts required for the hydrotalcite, and the nickel nitrate and stannous chloride metal salts required for the transition metal active component, using urea As an alkaline co-precipitation reagent, the initial pH of the mixed solution was measured to be about 3. Stir at 90-120°C for 5-20 hours, age the stirred mixed solution in a water bath at 25°C for 5-30 hours, and dry overnight at 80-120°C. The prepared hydrotalcite precursor is placed in a muffle furnace and calcined at 500-900° C. for 2-5 hours to obtain the Mg-Al-O composite oxide containing tin and nickel. The catalyst is reduced in a hydrogen stream at 300-600°C for 4 to 9 hours to obtain the dehydrogenation catalyst used in the process of the present invention, wherein the mass percentage of nickel is 5 to 40 wt%, and the mass percentage of ...
Embodiment 1
[0065] The preparation process of dehydrogenation catalyst C-1 is as follows: Dissolve 76.92 g of magnesium nitrate and 37.52 g of aluminum nitrate metal salt required for forming hydrotalcite with 350 ml of deionized water, and nickel nitrate required for introducing transition metal active components 8.6g and 2.5g of stannous chloride metal salt required for introducing main group metal components, adopt urea as alkaline co-precipitation reagent, the consumption of urea makes the initial pH of the mixed solution measured is about 3. Stirring at 100°C for 12 hours, the stirred mixed solution was aged in a water bath at 25°C for 20 hours, then filtered, and the filter cake was dried overnight at 105°C. The prepared dry hydrotalcite-based precursor was placed in a muffle furnace and calcined at 800°C for 3 hours to obtain a Mg-Al-O composite oxide containing tin and nickel. The catalyst was reduced in a hydrogen stream at 500°C for 6 hours to obtain the flake or layered catalys...
Embodiment 2
[0068] Example 1 was essentially repeated. The difference is that the reaction mixture obtained from the partial hydrogenation step of benzene (a mixture of benzene, cyclohexene and cyclohexane with 0.451 mole fractions of benzene, 0.122 mole fractions of cyclohexane, and The mole fraction is 0.427, based on the total molar weight of benzene, cyclohexane and cyclohexene), by selecting solid sulfonic acid resin as catalyst, first carry out addition reaction with formic acid, the temperature of addition reaction is 120 ℃, the reaction pressure 0.3 MPa to obtain a reaction mixture containing cyclohexyl formate, wherein the yield of ester was 99%. Then the resulting reaction mixture containing cyclohexyl formate was hydrolyzed by reactive distillation at 110°C, and separated during the reaction to obtain cyclohexanol, formic acid and light components respectively, of which cyclohexanol was The rate is 98.1%. The obtained light component is a mixture containing cyclohexene, cyclo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com