A kind of canine adenovirus type I inactivated vaccine and preparation method thereof
A technology of canine adenovirus and inactivated vaccines, applied in biochemical equipment and methods, viruses, vaccines, etc., to achieve good neutralizing antibody titers and excellent immune effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Serum-free culture process acclimatization of canine kidney cells (MDCK)
[0043] (1) Wash the canine kidney cells (MDCK) growing into a monolayer with pH 7.4 phosphate buffered saline solution twice;
[0044] (2) Add 0.25% trypsin for digestion, and discard the trypsin solution when the cells become round;
[0045] (3) Add MEM medium with a final concentration of 5% fetal bovine serum (v / v), add tylosin at a final concentration of 2-10 µg / mL, shake well, and place in a closed culture at 37°C for 24 hours;
[0046] (4) Set the culture temperature to 38.5°C and continue the airtight culture for 72 hours;
[0047] (5) The cells are covered with a monolayer, inoculated at a ratio of 1:10, and subcultured, and subcultured for 3 generations according to steps (1)-(4) to obtain seed cells;
[0048] (6) Wash the canine kidney cells (MDCK) growing into a monolayer twice with pH 7.4 phosphate buffered saline solution;
Embodiment 2
[0059] Example 2: Effects of various added factors on the proliferation characteristics of canine adenovirus type Ⅰ in serum-free culture
[0060] (1) Test material: Canine adenovirus type Ⅰ (CAV-Ⅰ) strain is CAV-ZY 180711, which I isolated and identified in 2018. For details, please refer to my published papers: Yi Cheng, Deng Li, Tang Qinghai et al., "Isolation and identification of a type I canine adenovirus", "China Animal Quarantine", Volume 36, Issue 2, 2019, pages 82-87, here, the applicant and the inventor guarantee that the The biological material can be released to the public within 20 years.
[0061] (2) Experimental design: Based on my previous research, I found that EDTA-Na 2 , taurine and cortisol respectively have a certain promoting effect on the proliferation of canine adenovirus type Ⅰ on MDCK cells. Therefore, in order to better carry out serum-free culture of canine adenovirus type Ⅰ, EDTA-Na 2 , taurine and cortisol are prepared into pro-virus proliferat...
Embodiment 3
[0069] Example 3: Proliferation characteristics of canine adenovirus type Ⅰ in serum-free culture
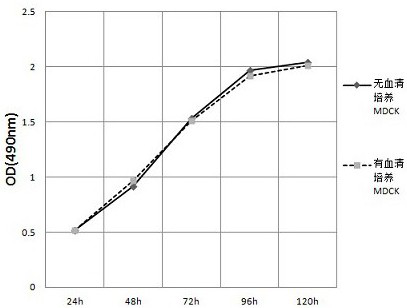
[0070] (1) Passage the serum-free acclimated MDCK cells obtained in Example 1 in the same way. When the confluence of the cells is about 90%, pour out all the culture medium in the spinner bottle, and add 0.9% (w / v) Wash the cells with 100 mL of NaCl washing solution, discard the washing solution, and repeat the washing once more.
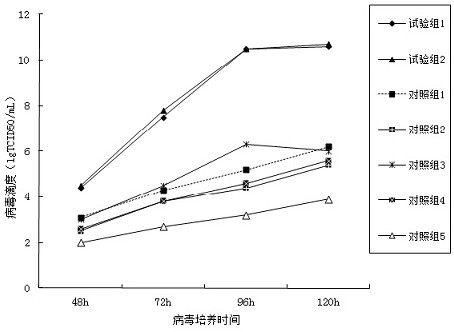
[0071] (2) Add the serum-free MEM medium obtained in Example 2-1 and its diluted canine adenovirus type 1 with a dilution factor of 1000 times, so that the virus multiplicity of infection is 1 TCID 50 , cultured in a spinner bottle with a rotating speed of 1 revolution / 5min, a culture temperature of 38.5°C, and airtight culture. Make 12 replicate samples, collect 3 replicate samples respectively at 48h, 72h, 96h and 120h after culture for TCID 50 determination.
[0072] (3) Serum-containing culture of canine adenovirus type Ⅰ was set up as a contr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com