An analytical method for the detection of impurities in telmisartan tablets and telmisartan capsules
A technique for analyzing telmisartan and an analysis method, which is applied in the analysis field of impurity detection of telmisartan tablets and telmisartan capsules, can solve problems such as poor baseline, affecting chromatographic column bonding, and impurity detection, and achieves saving Effects of detection cost, shortened analysis time, and reduced analysis cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
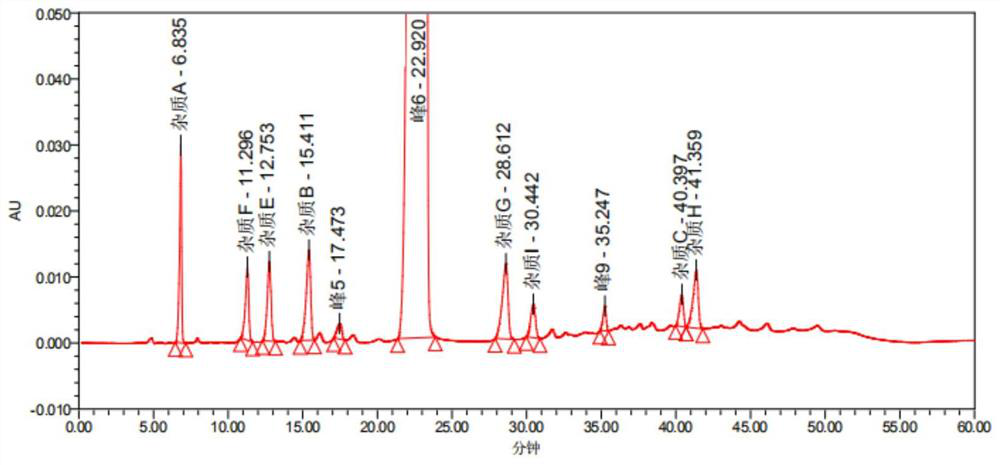
[0095] In order to further shorten the retention time of the main peak of telmisartan, increase the resolution between impurities, and reduce baseline fluctuations, the chromatographic conditions in Comparative Example 5 were optimized again, as shown below.
[0096] Instrument: Waters ACQUITY ARC (UHPLC)
[0097] Chromatographic column: C18, 250mm×4.6mm 3μm
[0098] Solution Ⅰ: Dissolve 2.0 g of potassium dihydrogen phosphate in an appropriate amount of water, adjust the pH to 3.0 with phosphoric acid, and add water to make 1 L;
[0099] Solution Ⅱ: Acetonitrile-Methanol (4:1)
[0100] Mobile phase A: solution Ⅰ-solution Ⅱ (80:20), ultrasonic degassing, that is;
[0101] Mobile phase B: solution Ⅰ-solution Ⅱ (20:80), ultrasonic degassing, that is;
[0102] Flow rate: 1mL / min Detection wavelength: 230nm
[0103] Column temperature: 40°C Injection volume: 20μl
[0104] The gradient elution program is shown in Table 3:
[0105] Table 3 Gradient elution program
[0106] ...
Embodiment 2
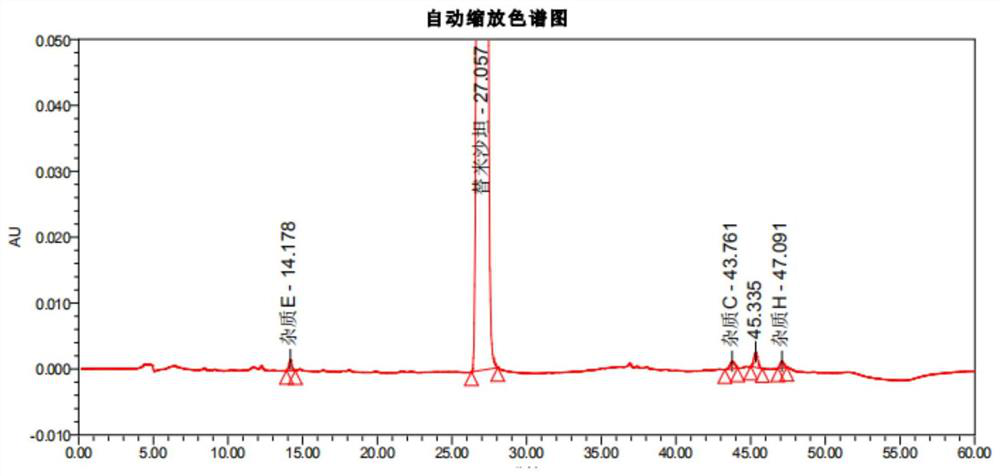
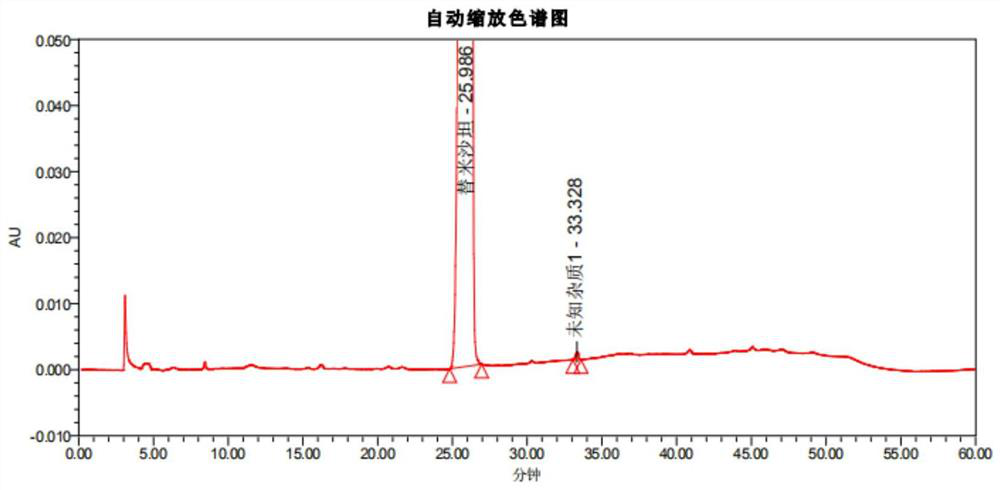
[0115] After methodological verification of the telmisartan impurity analysis method in the present invention, it was confirmed that the system applicability, specificity, quantitative limit and inspection limit, linearity and range, accuracy, repeatability and durability of the method all met the verification requirements; Then, the ultra-high performance liquid chromatography of the present invention is used to detect the related substances of the reference preparation and the self-developed telmisartan capsule.
[0116] Chromatographic conditions: with embodiment 1
[0117] Diluent: same as embodiment 1
[0118] Impurity contrast stock solution: with embodiment 1
[0119] System suitability solution: same as embodiment 1
[0120] Reference preparation solution: take an appropriate amount of telmisartan tablets-mecasu (644835, 644263, 644434), grind it finely, accurately weigh an appropriate amount of fine powder (approximately equivalent to telmisartan 50mg), put it in a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com