L-aspartate beta-decarboxylase mutants and application thereof
A technology of aspartic acid and decarboxylase, applied in the field of bioengineering, can solve problems such as long time, low specific enzyme activity, poor acid stability, etc., and achieve good enzymatic properties and improved acid stability of mutants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The construction of embodiment 1 recombinant escherichia coli
[0040] The Asd gene sequence was artificially synthesized (positions 1415236 to 1416837 of the gene whose NCBI accession number is AP019740.1), and specific primers P1 and P2 were designed (the underlined parts are the restriction endonuclease sites of EcoRI and XhoI, respectively).
[0041] Table 1 Primer list
[0042] P1 5'-CCG GAATTC ATGGGGAATGTAGATTATTCTAAAT-3'
P2 5'-CCG CTCGAG TCAGGACTCATCTTTTTTTAGTTCCC-3'
[0043] The L-aspartic acid β-decarboxylase gene Asd was digested by EcoRI and XhoI and connected to the expression vector pET 28a(+) digested with the same restriction enzyme to obtain the recombinant plasmid pET 28a-ArAsd.
[0044] Existing studies have shown that changing the charged amino acid residues on the surface of the enzyme molecule has a certain effect on the optimum reaction pH of the enzyme (Alan J. Russell A R F. Rational modification of enzyme catalysi...
Embodiment 2
[0053] Example 2 Expression and purification of L-aspartate β-decarboxylase
[0054] BL21 / pET28a-N35D, BL21 / pET28a-A179E recombinant Escherichia coli were inoculated in 5 mL of LB medium with a kanamycin concentration of 50 μg / mL, and cultured overnight at 37°C with shaking at 200 rpm. The above overnight culture was inoculated into 2YT medium containing 50 μg / mL of kanamycin at an inoculum size of 1%, and cultured with shaking at 37°C and 200 rpm until the bacterial liquid OD 600 To 0.6-0.8, add IPTG to a final concentration of 0.2mmol / L, induce culture at 20°C for about 20h to obtain bacteria. After collecting the bacteria by centrifugation at 6000rpm, they were ultrasonically crushed, and the protein was purified using a His Trap HP affinity column, and the target protein was detected by SDS-PAGE. The results were as follows: figure 1 shown. The specific enzyme activity of the pure enzyme was measured, and the specific enzyme activity of the enzyme mutants N35D and A179E ...
Embodiment 3
[0055] Example 3 pH Stability Determination
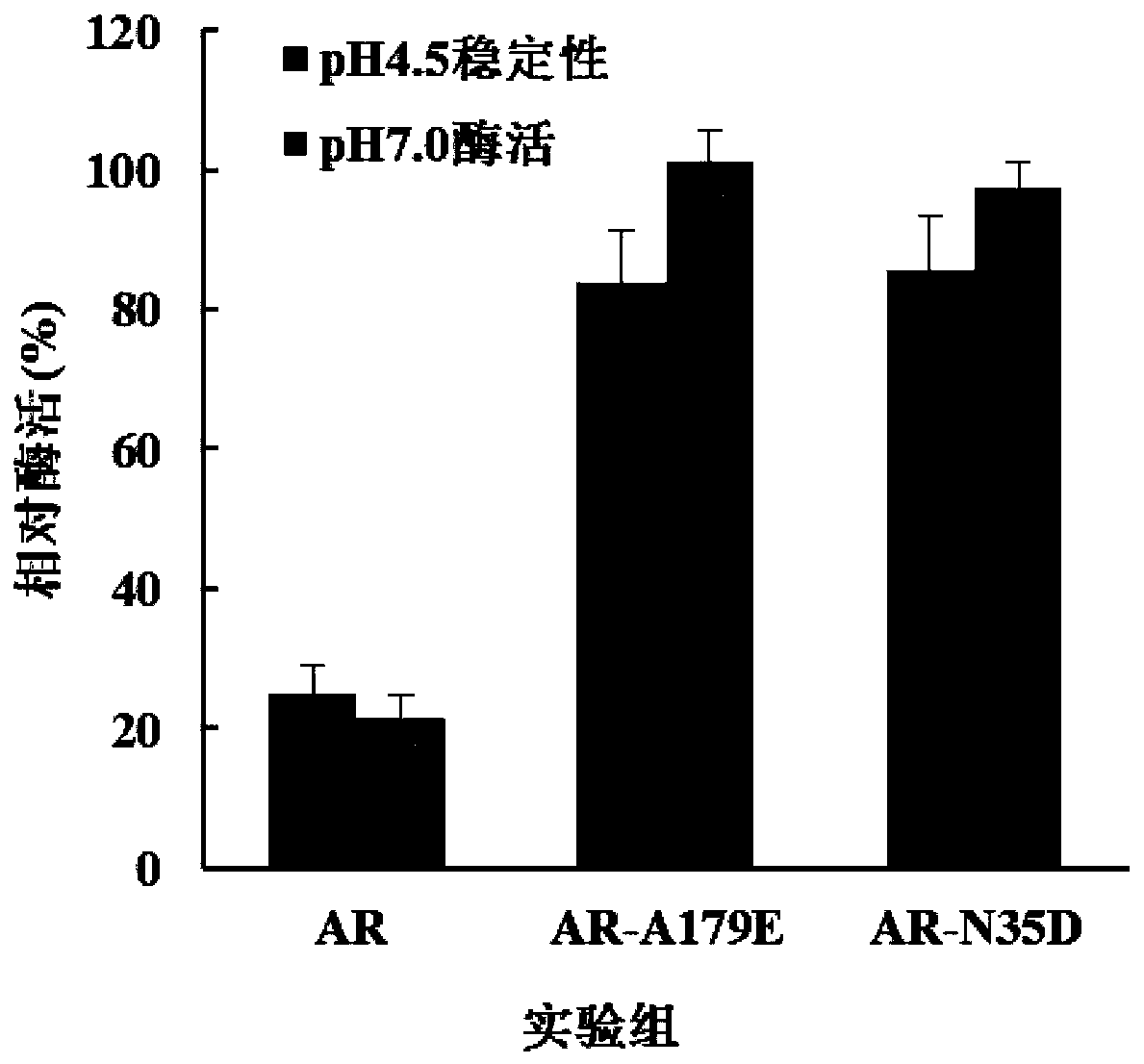
[0056] Such as figure 2 As shown, the enzymatic activity of L-aspartic acid β-decarboxylase mutants N35D and A179E increased by 238% and 244% respectively under the condition of pH 7.0.
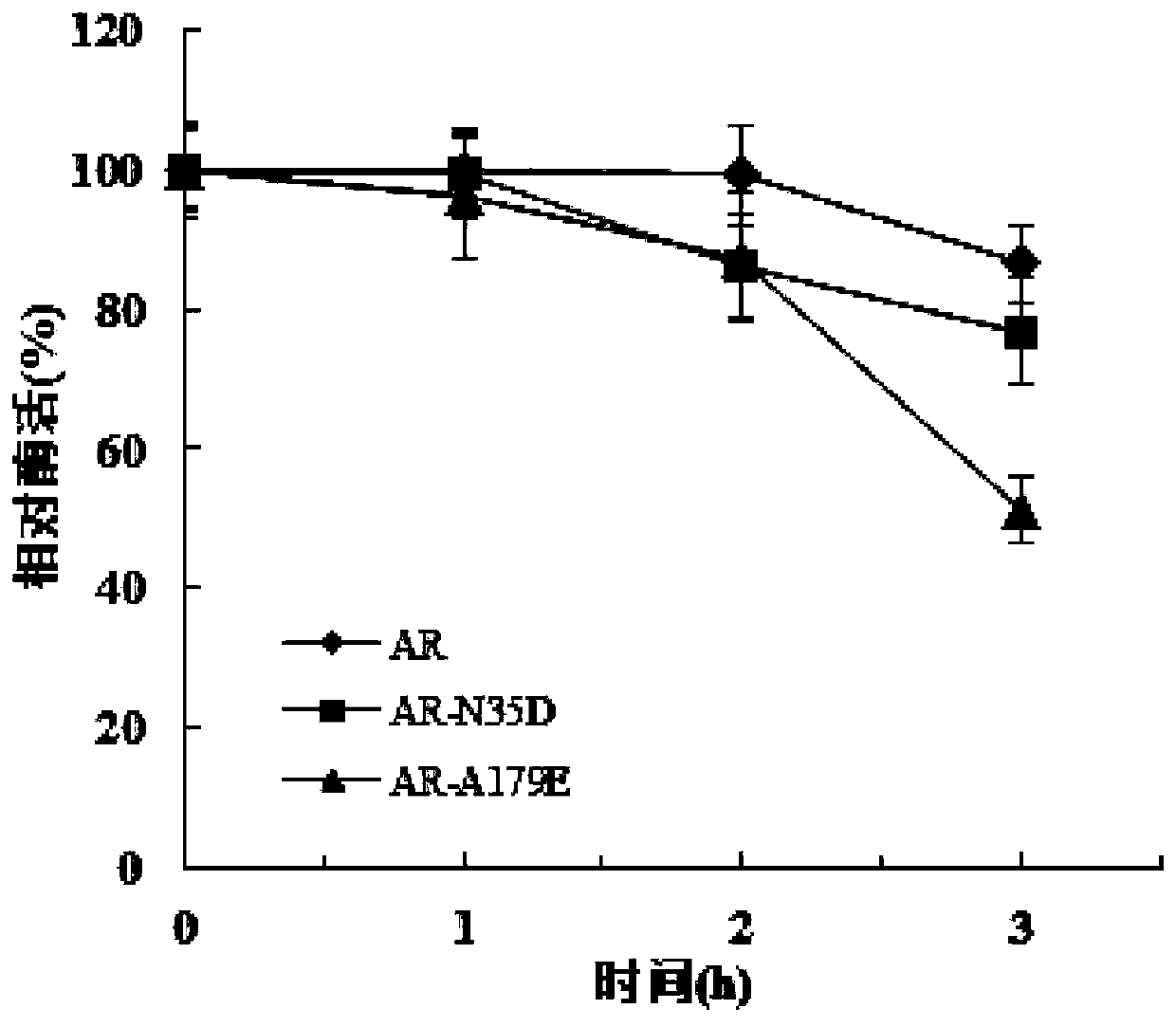
[0057] After 12 hours of treatment at pH 4.5, the residual enzyme activities of N35D and A179E mutants remained 83.9% and 85.5%, respectively, compared with 24.8% of the wild-type enzyme, and the acid stability of the mutants was significantly improved.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com