Construction and application of core-shell type intelligent nano delivery system
A technology of chondroitin sulfate and chlorin, which is applied in the synthesis of chondroitin sulfate-chlorin amphiphilic polymer, the application field of core-shell type cross-linked self-assembled nano drug delivery system combined with ultrasonic therapy for anti-tumor , can solve the problem of less application, and achieve the effect of improving aggregation, inducing cell apoptosis, and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
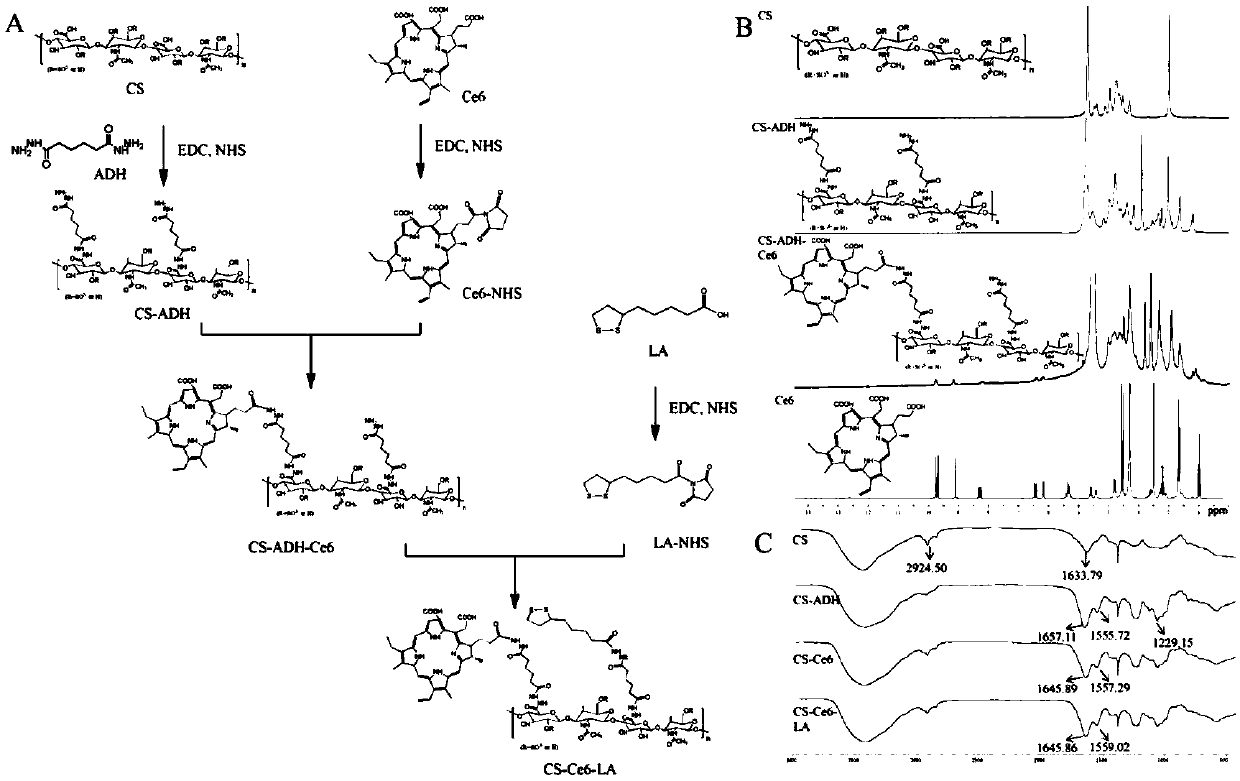
[0052] In another embodiment of the present invention, the preparation method of the chondroitin sulfate-chlorin e6 amphoteric polymer modified by lipoic acid is provided, and the steps of the preparation method are as follows:
[0053] (1) Synthesis of chondroitin sulfate-adipate dihydrazide: Dissolve chondroitin sulfate in distilled water, add adipate dihydrazide, 1-(3-dimethylaminopropyl) -3-Ethylcarbodiimide hydrochloride, N-hydroxysuccinimide, adjust the pH of the reaction system to 6-7 with sodium hydroxide, and stir at room temperature for 20-30 hours to obtain chondroitin sulfate-adipic acid Dihydrazide;
[0054] (2) Activation of chlorin e6: Dissolve chlorin e6 in N,N-dimethylformamide, and sequentially add 1-(3-dimethylaminopropyl)-3-ethyl carbon Diimine hydrochloride, N-hydroxysuccinimide, activated at room temperature for 5-8 hours, then dropwise adding an organic base to adjust the pH of the solution to 7-9;
[0055] (3) Synthesis of chondroitin sulfate-chlorin ...
Embodiment 1
[0074] Example 1: Synthesis of lipoic acid-modified chondroitin sulfate-chlorin e6 polymer
[0075] (1) Synthesis of chondroitin sulfate-adipate dihydrazide: Weigh 0.5g chondroitin sulfate and dissolve it in 150mL distilled water, stir to make it fully swell and dissolve, then add 5.0g adipic acid dihydrazide to the solution in turn Hydrazide, 1.6g 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and 0.23g N-hydroxysuccinimide, adjust the pH value of the reaction system to 6.8 with sodium hydroxide , reacted at room temperature for 24 hours, dialyzed with distilled water for three days, and freeze-dried to obtain the intermediate product chondroitin sulfate-adipate dihydrazide.
[0076] (2) Weigh 12 mg of chlorin e6 and dissolve it in 4 mL of N,N-dimethylformamide, and sequentially add 1-(3-dimethylaminopropyl )-3-ethylcarbodiimide hydrochloride, N-hydroxysuccinimide equivalent to 2 times the molar amount of chlorin e6, stirred at room temperature for 30 minutes, a...
Embodiment 2
[0078] Embodiment 2: the synthesis of chondroitin sulfate-chlorin e6 polymer
[0079] (1) Synthesis of chondroitin sulfate-adipate dihydrazide: Weigh: 0.5g chondroitin sulfate is dissolved in 150mL distilled water, stir to make it fully swell and dissolve, then add 5.0g adipic acid to the solution successively Dihydrazide, 1g 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and 0.1g N-hydroxysuccinimide, adjust the pH value of the reaction system to 6.6 with sodium hydroxide , reacted at room temperature for 24 hours, dialyzed with distilled water for three days, and freeze-dried to obtain the intermediate product chondroitin sulfate-adipate dihydrazide.
[0080] (2) Weigh 24 mg of chlorin e6 and dissolve it in 3 mL of N,N-dimethylformamide, and sequentially add 1-(3-dimethylaminopropyl )-3-ethylcarbodiimide hydrochloride, N-hydroxysuccinimide equivalent to 2 times the molar amount of chlorin e6, stirred at room temperature for 30 minutes, and then added 2 times th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com