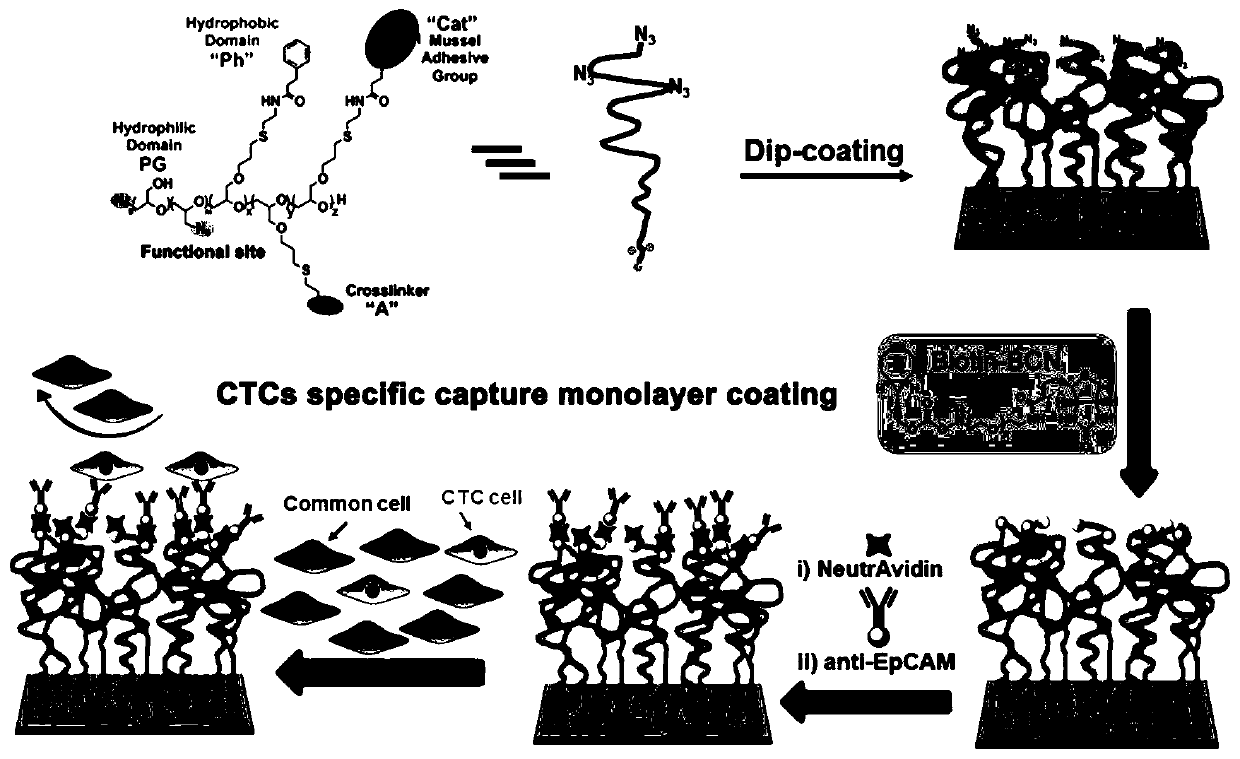
Cancer monitoring-oriented biological coating for specific whole blood capture of circulating tumor cells and preparation method of biological coating
A biological coating and tumor cell technology, applied in the field of functional material technology and biomedical materials, can solve the problems of complex material preparation process, long enrichment time, and low specificity of cell capture, and achieve efficient capture, high capture efficiency, The effect of efficient and sensitive capture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Dehydrate tetra-n-octyl ammonium bromide, add ethoxy ethyl glycidyl ether and triisobutyl aluminum to toluene, react at 0°C for three hours, and then add allyl under ice bath conditions Glycidyl ether and triisobutylaluminum were stirred overnight at room temperature. Add water to terminate the reaction, dry the polymer product with sodium sulfate, remove toluene, and purify by dialysis to obtain the polymer (PEEGE-b-PAGE).
[0041] (2) Add the polymer (PEEGE-b-PAGE) obtained in step (1) into tetrahydrofuran together with 37% hydrochloric acid aqueous solution, stir and react at room temperature for 10 hours, wash the polymer precipitate with tetrahydrofuran, and dialyze with methanol to obtain the Acetal protected polymer (PG-b-PAGE).
[0042] (3) Dissolve the polymer (PG-b-PAGE) obtained in step (2) in dry N,N-dimethylformamide, add triethylamine under ice bath conditions, and then add p-toluene Sulfonyl chloride was reacted overnight with stirring at room tempe...
Embodiment 2
[0058] Step (1)-step (13) is identical with embodiment 1.
[0059] (14) Use Accutase to digest MCF7 / T47D / MDA-MB-231 / A549 / HeLa from the cell culture flask, and use CellTrace TM Cells were pre-stained with violet dye for 20 minutes, and the stained cells were mixed into serum-free RPMI-1640 medium at a concentration of 5000 cells per milliliter to make a cancer cell suspension.
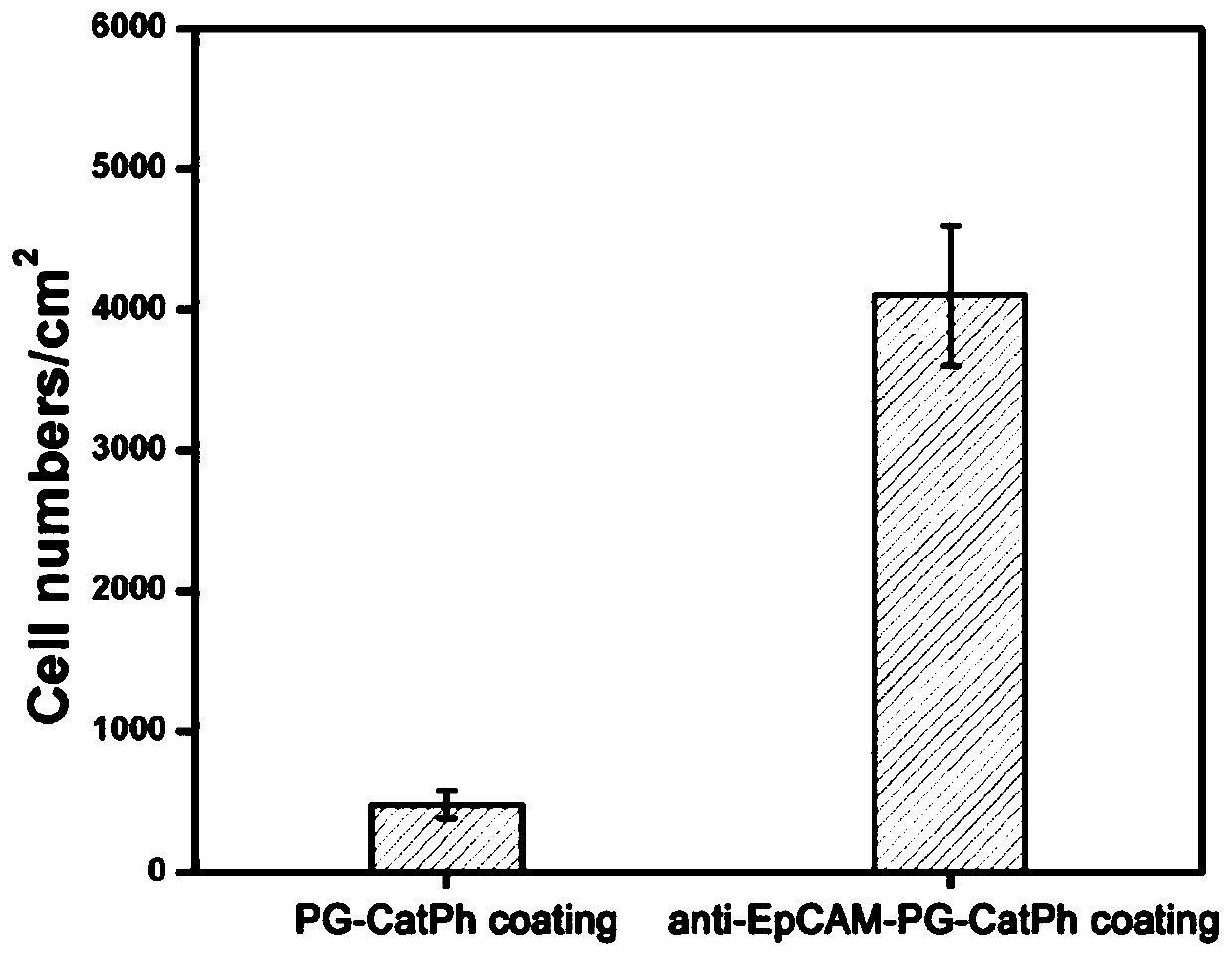
[0060] (15) Place the Anti-EpCAM-PG-CatPh substrate obtained in step (13) face up in an eight-hole plate, add 1 ml of breast cancer cell MCF7 suspension with a concentration of 5000 per ml, and place in cell culture In the case (preferred reaction time is 90 minutes), breast cancer cell MCF7 suspension is removed, the bio-substrate is fully rinsed in phosphate buffer, and photographed respectively under 10 times with a Zeiss fluorescence microscope (at least 3 substrates each time) , each substrate selects 10 different positions in the middle), and counts the breast cancer cells MCF7 captured on the b...
Embodiment 3
[0067] Step (1)-step (13) is identical with embodiment 1.
[0068] (14) Use Accutase to digest MCF7 / A549 / MDA-MB-231 / HeLa from the cell culture flask, and use CellTrace TM Cells were prestained with violet dye for 20 minutes. Whole blood from healthy donors was incubated with erythrocyte lysate on ice for 10 minutes to lyse red blood cells, and then the pre-stained MCF7 cells were mixed into healthy donor blood at a concentration of 1000 cells per milliliter to make artificial breast cancer patient blood samples / Artificial blood samples of lung cancer patients / Artificial blood samples of cervical cancer patients.
[0069](15) Place the Anti-EpCAM-PG-CatPh substrate obtained in step (13) face up in an eight-hole plate, add 1 milliliter of artificial breast cancer patient blood samples with a concentration of 1000 per milliliter, and place in a cell culture incubator (preferred reaction time is 90 minutes), remove the artificial breast cancer patient blood sample, the bio-sub...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com