A kind of immobilization method of Candida antarctica lipase b
A technology of Candida Antarctica and lipase, applied in the field of enzyme engineering, can solve problems such as loss, and achieve the effects of convenient operation, good temperature stability and solvent tolerance, and simple preparation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Immobilization conditions and lipase activity assay method of Candida antarctica lipase B
[0024] Add dimethylimidazole and zinc nitrate to deionized water respectively, and sonicate at 40 Hz for 20 minutes to prepare 0.3125 mol / L dimethylimidazole aqueous solution and 0.3125 mol / L zinc nitrate aqueous solution respectively. Take 60mL0.3125mol / L dimethylimidazole aqueous solution, 1.5mL0.3125mol / L zinc nitrate aqueous solution and 3mL Candida antarctica lipase B enzyme solution (Novozymes, Denmark) CALB, enzyme amount 72mg / ml, 4166.67U / ml) were mixed to obtain a mixed enzyme liquid, and reacted with magnetic stirring at 25°C and 200rpm for 30min to obtain a reaction liquid containing MOFs (CALB-ZIF-8) complex. Add 0.5g of macroporous resin D101-1 and 2.5mL of 25% polyvinylimide (PEI) aqueous solution to the reaction solution, conduct crosslinking reaction at 30°C and 200rpm in a water bath for 5 hours, and vacuum After suction filtration, the filter cake wa...
Embodiment 2
[0031] The ratio and the concentration of the solution used in the preparation process of the immobilized enzyme of embodiment 2 have an impact on the enzyme activity
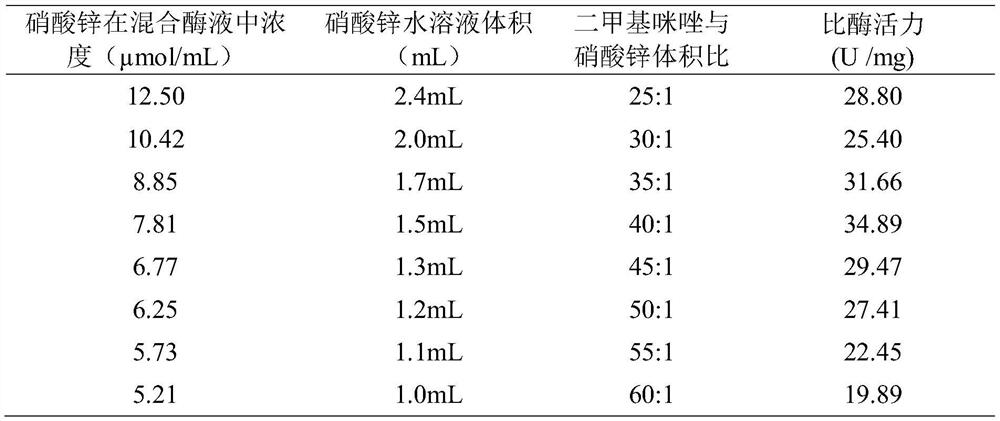
[0032] Zinc nitrate aqueous solution volume is set as 1.0mL-2.4mL among the embodiment 1, other operation and condition are identical with embodiment 1, and the result is as shown in table 1, and the optimum volume ratio of dimethylimidazole aqueous solution and zinc nitrate aqueous solution is 40: 1.
[0033] Table 1: Specific enzyme activity results of different volumes of zinc nitrate aqueous solution
[0034]
Embodiment 3
[0035] The impact of the amount of enzyme liquid used in the preparation process of embodiment 3 immobilized enzyme on enzyme activity
[0036] The volume of Candida antarctica lipase B enzyme liquid in Example 1 is set to 1-6ml, other operations and conditions are the same as in Example 1, the results are as shown in Table 2, the optimal value of adding enzyme liquid is 3mL (72mg / ml).
[0037] Table 2 The specific enzyme activity results of different volumes of enzyme solution
[0038]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com